Outcomes in Kidney Transplant Recipients after Conversion from Tacrolimus to Belatacept-Based Regimens
Columbia University Irving Medical Center, New York, NY
Meeting: 2019 American Transplant Congress
Abstract number: A265
Keywords: Co-stimulation, Infection, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
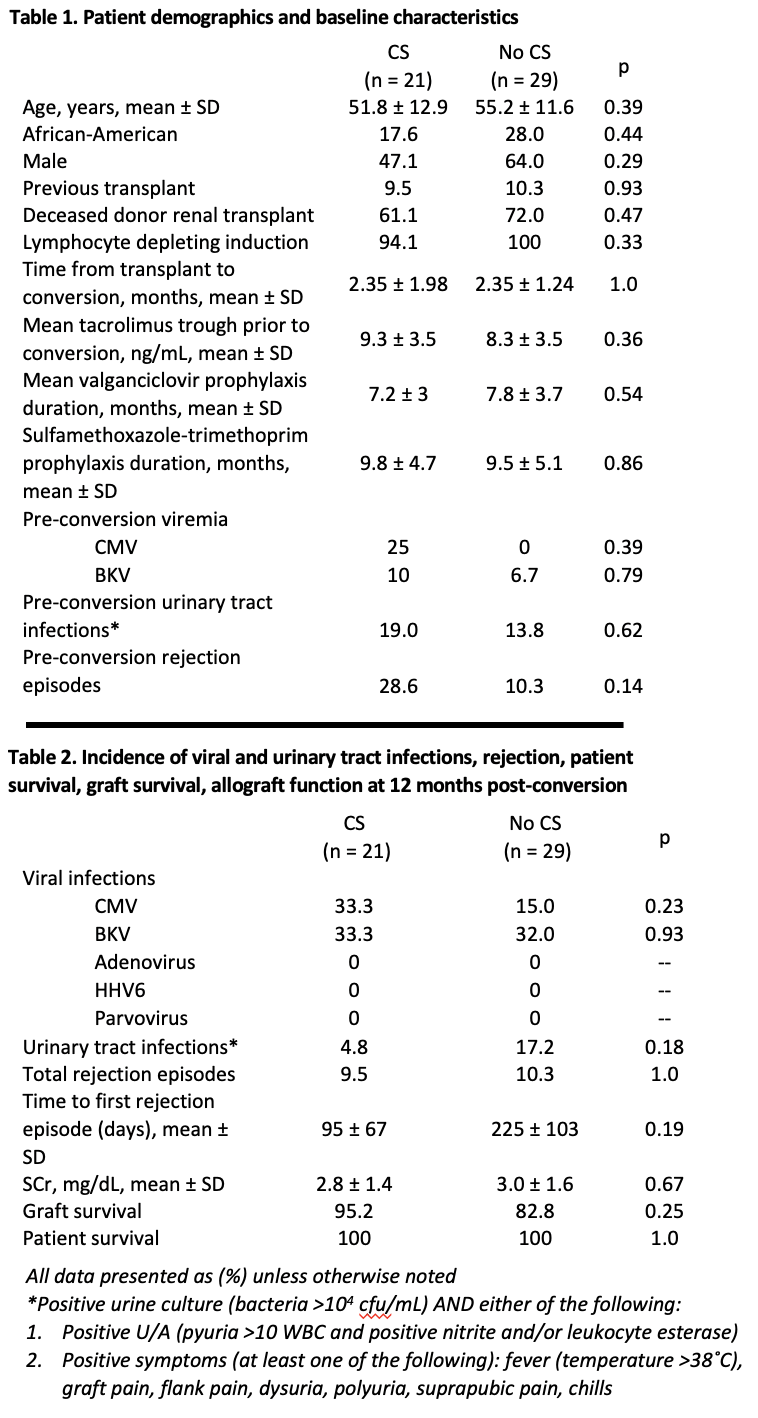
*Purpose: Immunosuppressive strategies for belatacept are variable and associated outcomes have not been well described. We examined outcomes among kidney transplant (KT) recipients converted from tacrolimus to belatacept-based regimens with or without corticosteroids (CS).
*Methods: Retrospective study of adult patients who underwent KT between 02/2014 and 11/2017 and were converted from tacrolimus to belatacept-based regimens within 6 months post-transplant. Patients were stratified on whether conversion was accompanied with newly initiated CS therapy. Outcomes of interest included rejection, kidney function, infections, and patient and allograft survival at 12-months post-conversion.
*Results: We identified 50 patients who were converted from tacrolimus to belatacept maintenance immunosuppression during the study period; 21 (42%) were converted with newly initiated CS. Following conversion, rejection was similar in the CS group (9.5%) compared to the non-CS group (10.3%) (p=1.000). Mean SCr at 12 months post-conversion was 2.8 ± 1.4 mg/dL and 3.0 ± 1.6 mg/dL in the CS and non-CS groups, respectively (p=0.670). There were no differences in the incidence of viral or urinary tract infections. Patient survival was 100% in both groups at 12 months post-conversion, and there was no difference in graft survival 95.2 vs 82.8% (p=0.246) (Table 2).
*Conclusions: Patients undergoing early (within 6 months of KT) conversion to a belatacept-based regimen with CS experienced a similar number of rejection and infection episodes compared to those converted without CS. Further study of larger populations to evaluate belatacept conversion regimen outcomes are needed.
To cite this abstract in AMA style:
Underwood H, Lange N, Hedvat J, Brennan C, Tsapepas D, King K, Husain S, Salerno D, Patel S, Mohan S, Crew R. Outcomes in Kidney Transplant Recipients after Conversion from Tacrolimus to Belatacept-Based Regimens [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-in-kidney-transplant-recipients-after-conversion-from-tacrolimus-to-belatacept-based-regimens/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress