Outcomes After Conversion to Belatacept in Pediatric Kidney Transplantation
Pediatric Nephrology, Robert Debré Hospital, APHP, Paris, France
Meeting: 2022 American Transplant Congress
Abstract number: 828
Keywords: Co-stimulation, Kidney, Outcome, Pediatric
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Belatacept (CTLA-Ig) is a selective co-stimulation blocker that is associated with reduced dnDSA, improved renal function, and prolonged allograft survival in adult transplant recipients compared to calcineurin inhibitor (CNI) based regimens. Its use in older children and young adults is limited. We report outcomes for 13 pediatric patients converted to belatacept.
*Methods: All 13 patients converted to belatacept therapy between 05/2018 and 01/2021 in Robert Debré Hospital, Paris were included. Patients received an induction with basiliximab (n=8) or antithymocyte globulin (n=5). Maintenance immunosuppression included CNI, antimetabolite and steroids. Patients’ viral status (EBV, CMV) were monitored monthly and allograft biopsy was performed prior to conversion and 6 months after conversion. The first 5 belatacept injections were administered at 5mg/kg/dose every 2 weeks, then monthly. CNI doses were decreased by 25% at each infusion and stopped after 2 months. Antimetabolite doses were also increased at CNI withdrawal. 6/13 patients were steroid-free at the time of conversion.
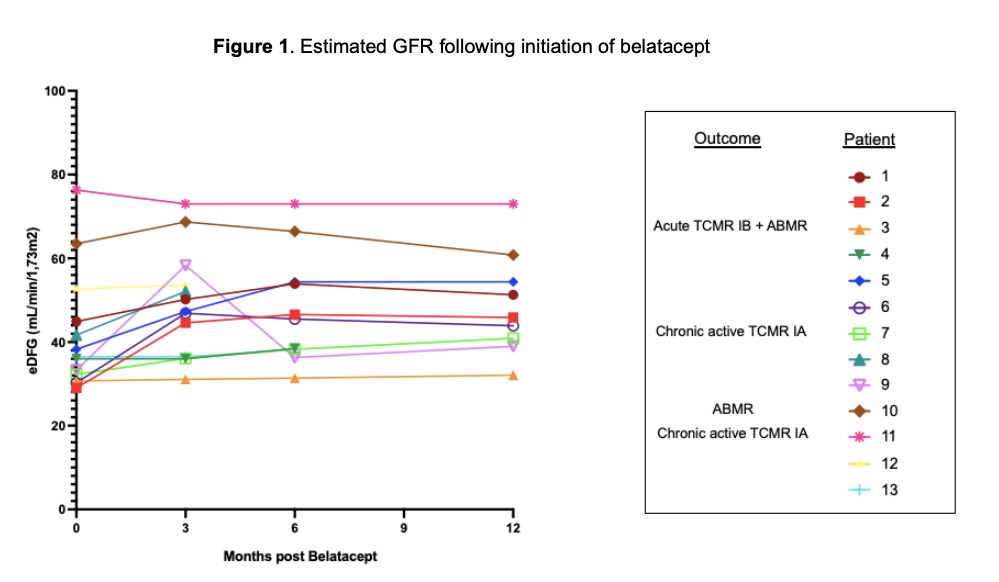
*Results: The median age at conversion was 17,6 years (range 10,3-19,4) and 3,9 years (IQR 1,3 – 6) post-transplant. Conversion indication was based on medical need for long term CNI avoidance (side-effect or histological evidence of CNI toxicity, n=11) or to improve adherence (e.g. monthly IV-treatment, n=2). CNI was withdrawn in all patients by a median of 42 days (IQR 42-75) after starting belatacept. GFR was stable or improved over a median follow-up time of 12,1 months (IQR 9,6-20,3), Figure 1. Rejection episodes were observed in 4/13 patients (median 10,3 months, IQR 7,3-19,1): 2 chronic active TCMR IA, 1 mixed acute rejection (TCMR IB and ABMR) and 1 ABMR (both DSA-negative). Among these patients, 2 had prior history of rejection (with normal pre-belatacept biopsies), 1 showed minimal interstitial inflammation without tubulitis (and was off steroids) prior to starting belatacept and 1 had been converted for adherence problems, which subsequently persisted. These rejection episodes showed good evolution after treatment in all patients, but CNI were reintroduced for two of them. Regarding all the other patients, no severe viral complications or development of dnDSA were observed. Four patients had pre-existing DSA which remained stable.
*Conclusions: Selected pediatric kidney recipients may benefit from long-term CNI toxicity avoidance, but selection criteria need to be refined to avoid rejection episodes under costimulation blockade.
To cite this abstract in AMA style:
Duneton C, Maisin A, Cheyssac E, Dahdouh H, Baudouin V, Hogan J. Outcomes After Conversion to Belatacept in Pediatric Kidney Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-after-conversion-to-belatacept-in-pediatric-kidney-transplantation/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress