Once Weekly Fluconazole is Effective for the Prevention of Oral Candidiasis in Kidney Transplant Recipients
J. Lockridge, D. Roberts, K. Spence, M. Stack, S. Rehman, A. Olyaei
OHSU, Portland, OR
Meeting: 2020 American Transplant Congress
Abstract number: A-202
Keywords: Fungal infection, Kidney, Pharmacoeconomics, Prophylaxis
Session Information
Session Name: Poster Session A: Kidney Infectious Excluding Polyoma & Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Oropharyngeal candidiasis is an infection prevalent in renal transplant recipients, with significant concerns for invasive endoscopic workup and development into invasive fungal infections. While clotrimazole and nystatin remain options for prophylaxis, these agents are often cumbersome to administer and have recently been subject to drug shortages. An alternative option is oral fluconazole, which is particularly appealing when dosed on a once weekly basis to avoid drug-drug interactions. The purpose of this study is to assess the efficacy in prevention of oropharyngeal candidiasis in kidney transplant recipients with once weekly fluconazole.
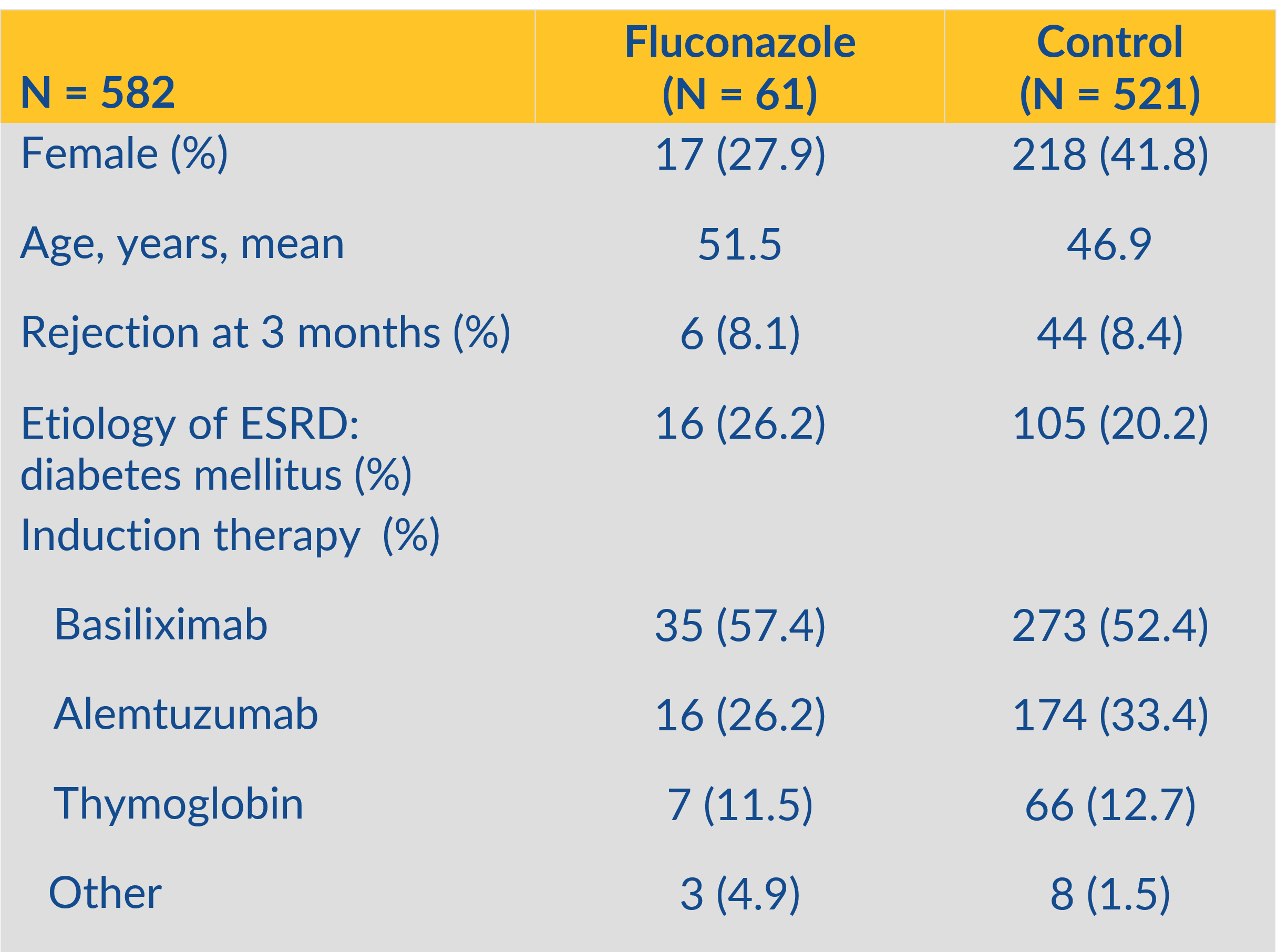
*Methods: This institutional review board-approved retrospective case control study was conducted by evaluating the electronic health records of kidney transplant recipients at a single center between March 1st, 2014 and January 31st, 2019. The once weekly fluconazole treatment group consisted of 61 patients, compared with 521 patients in the control (no prophylaxis) group. Patients who received anti-fungal prophylaxis with agents other than fluconazole were excluded. The primary outcome was incidence of oropharyngeal candidiasis after use of once weekly oral fluconazole 400 mg following kidney transplant.
*Results: A total of 582 kidney transplant recipients who underwent transplantation were included in the analysis. Patients were followed for a total of 90 days from transplant and the incidence of oral candidiasis was documented. Sixty-one patients [12.7%] in the control group (no prophylaxis) developed proven/probable oral candidiasis which required treatment with nystatin or daily doses of fluconazole. Twenty two percent of these patients had recurrence and/or vaginal candidiasis in addition to the initial episode of oral candidiasis. In patients receiving fluconazole 400 mg orally weekly, none experienced oral candidiasis (P< 0.001). The average tacrolimus level was 7.8 ng/dl in the fluconazole group and 8.5 ng/dl in the control group. No fungal resistance was observed.
*Conclusions: Once weekly oral fluconazole is effective and safe to reduce the incidence of oropharyngeal candidiasis. While potentially effective in preventing fungal infections, fluconazole weekly 400 mg use was not associated with supratherapeutic tacrolimus levels or a higher rate of inherently resistant fungal infections. Further clinical trials are warranted to confirm the clinical significance of these effects.
To cite this abstract in AMA style:
Lockridge J, Roberts D, Spence K, Stack M, Rehman S, Olyaei A. Once Weekly Fluconazole is Effective for the Prevention of Oral Candidiasis in Kidney Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/once-weekly-fluconazole-is-effective-for-the-prevention-of-oral-candidiasis-in-kidney-transplant-recipients/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress