Obesity Impacts Acute Rejection but Not Allograft Function Improvement in the Setting of Belatacept Conversion
University of Illinois at Chicago, Chicago, IL
Meeting: 2021 American Transplant Congress
Abstract number: 941
Keywords: Co-stimulation, Kidney transplantation, Obesity, Rejection
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: There is limited data regarding belatacept conversion in obese patients as an alternative to calcineurin inhibitor (CNI) immunosuppression. Pharmacokinetic (PK) data suggests increasing clearance and volume of central/peripheral compartments with increasing body weight. The purpose of this study is to compare rejection, allograft function, and infectious outcomes of obese versus non-obese renal transplant (RT) recipients converted to belatacept.
*Methods: Adult RT recipients between 3/1/2018 – 5/30/2020 at the University of Illinois Hospital & Health Sciences System converted to belatacept were assessed. Patients who received multiorgan transplants or were lost to follow up were excluded. Belatacept was initiated at 10mg/kg on days 1, 5, 14, 28, and then every 4 weeks until week 16 where patients received 5mg/kg every 4 weeks. CNIs were tapered to discontinuation over 8 weeks. All patients were started on prednisone 10mg at conversion. Obese (body mass index [BMI] > 35 kg/m2) and non-obese patients were compared. High immunologic risk was defined as PRA > 10%, presence of DSA at RT, and positive XM. Acute rejection (defined as biopsy proven acute rejection (BPAR) or empiric rejection treatment) was compared. Allograft function (measured by eGFR), BK viremia, CMV viremia, death-censored allograft survival, and patient death were also analyzed.
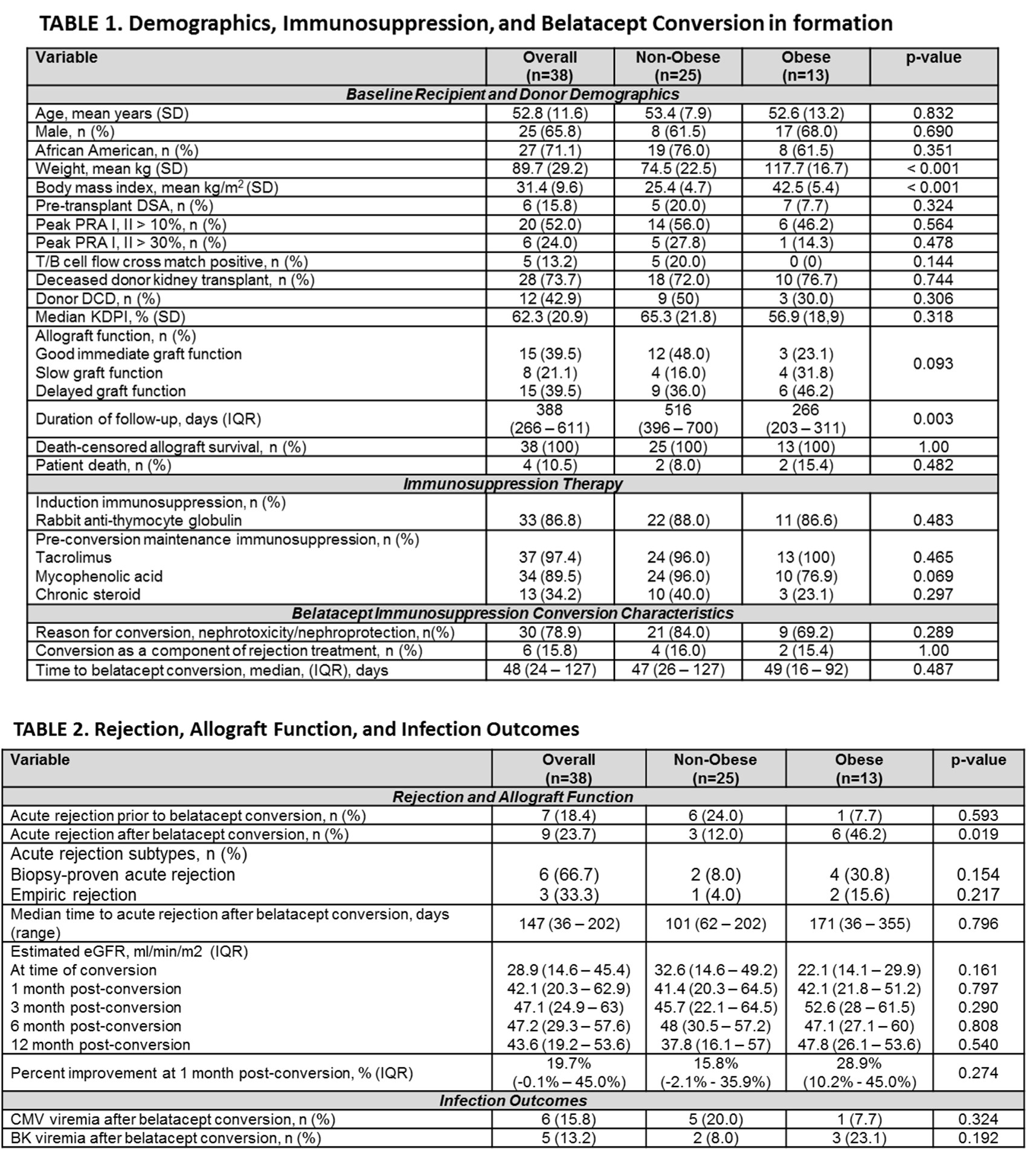
*Results: A total of 38 RT recipients were included (25 [65.7%] non-obese patients and 13 [34.2%] obese patients (Table 1). Belatacept conversion occurred at a median of 48 days (IQR 24 – 127 days) post-transplant for predominantly nephrotoxicity/nephroprotection (78.9%). Acute rejection was significantly higher in obese patients after belatacept conversion (non-obese 12% vs obese 46.2%, p=0.019). Table 2 details rejection and allograft function. Figure 1 illustrates time to acute rejection survival curve analyses. Despite this there was no difference in eGFR. Incidence of post-transplant viremias were similar (Table 2).
*Conclusions: Acute rejection was observed more frequently in the obese RT cohort without impact to allograft function. More studies examining the PK and clinical outcomes of belatacept in obese patients is warranted.
To cite this abstract in AMA style:
Pierce D, Benken J, West-Thielke P, Tzvetanov I, Benedetti E, Lichvar A. Obesity Impacts Acute Rejection but Not Allograft Function Improvement in the Setting of Belatacept Conversion [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/obesity-impacts-acute-rejection-but-not-allograft-function-improvement-in-the-setting-of-belatacept-conversion/. Accessed February 20, 2026.« Back to 2021 American Transplant Congress