National Consortium Registry Insights on Management and Outcome of Hepatitis C Viremic Heart Transplants
R. Gottlieb1, M. Salem1, A. Ravichandran2, J. Kennedy3, J. Strope4, S. Lin5, J. Chow6, P. Eckman7, S. Desai8, O. Larkins1, J. Vanzyl1, S. Hall1
1Baylor Scott & White Healthcare, Dallas, TX, 2Ascension Medical Group, Indianapolis, IN, 3Inova Heart and Vascular Institute, Falls Church, VA, 4Medical University of South Carolina, Charleston, SC, 5University of Washington Medical Center, Seattle, WA, 6Tufts Medical Center, Boston, MA, 7Minneapolis Heart Institute, Minneapolis, MN, 8Ochsner Medical Center, New Orleans, LA
Meeting: 2022 American Transplant Congress
Abstract number: 557
Keywords: Heart transplant patients, Hepatitis C, Infection, Outcome
Topic: Clinical Science » Infection Disease » 27 - Non-Organ Specific: Viral Hepatitis
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:10pm-6:20pm
Presentation Time: 6:10pm-6:20pm
Location: Hynes Room 312
*Purpose: Multicenter US registry of clinical management of HCV NAT+ hearts.
*Methods: An ongoing 8-center retrospective IRB-approved registry collected baseline characteristics, labs, management, and outcomes after isolated HCV NAT+ heart transplant (HT).
*Results: The 129 HCV NAT+ HT incidence was linear with time. Recipient median age 57y (IQR 49, 64) from donors in 10 of 11 UNOS regions. Transplant was repeat sternotomy for 59% (n=76). Of 110 HCV-viremic HT under new UNOS allocation system (effective 10/18/2018), recipients at listing status 1-6 were 5% (status 1), 35%, 26%, 28%, 2%, and 4% (status 6), respectively. Of 101 patients evaluable at 1 year, survival was 94% (n=95) and 6% expired (n=6).
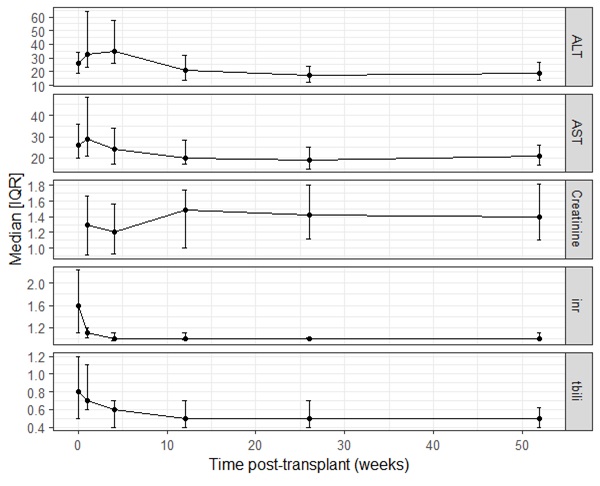
63% (n=81) were transplanted from mechanical circulatory support (MCS). Of these, 63% (n=51) were supported on outpatient compatible devices, 36% (n=29) included temporary MCS and 5% (n=4) from ECMO. Renal & hepatic lab trends are shown (Fig 1).
Direct-acting antiviral (DAA) was used to treat 86% (n=111) of patients, most commonly pangenotypic glecaprevir/pibrentasvir (Mavyret, 64%, n=71), sofosbuvir/velpatasvir (Epclusa, 34%, n=38), or genotype-restricted ledipasvir/sofosbuvir (Harvoni, 2%, n=2).
DAA payor was known for 82/111 (74%). Commercial insurance was most common (50%, n=41), followed by Medicare (22%, n=18), TROJAN-C trial (15%, n=12), Medicaid (12%, n=10), and 1 via the VA.
94 recipients used statin post-transplant (73%). Of these, the most common statins were pravastatin (78%), atorvastatin (16%), and rosuvastatin (6%). Only 40% (35/93) had statin dose adjusted on DAA treatment. PPI was used for ≥72% of patients; when used, PPI was typically not interrupted (continued in 78%, 73/93; interrupted 5%, 5/93; unknown 16%,15/93).
Immunosuppression patterns and cure-rate (SVR-12) will also be presented.
*Conclusions: A multicenter consortium registry provides additional insights into HCV-viremic heart transplants. Pangenotypic regimens are common. Statin and PPI use was typically non-limiting.
To cite this abstract in AMA style:
Gottlieb R, Salem M, Ravichandran A, Kennedy J, Strope J, Lin S, Chow J, Eckman P, Desai S, Larkins O, Vanzyl J, Hall S. National Consortium Registry Insights on Management and Outcome of Hepatitis C Viremic Heart Transplants [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/national-consortium-registry-insights-on-management-and-outcome-of-hepatitis-c-viremic-heart-transplants/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress