Multi-Center, Open-Label Trial of Pre-Emptive Glecaprevir/Pibrentasvir (G/P) to Treat Recipients of Transplanted Kidneys from Deceased Donors with Hepatitis C Virus (MYTHIC)
M. Sise1, D. Goldberg2, J. Kort3, D. Schaubel4, R. Alloway5, C. Durand6, R. Fontana7, J. Friedewald8, R. Brown9, S. Prenner10, R. Landis4, P. Reese11, R. Chung1
1Internal Medicine - Nephrology, MGH, Boston, MA, 2Hepatology and Transplantation, U Miami, Miami, FL, 3Abbvie, Chicago, IL, 4Department of Biostatistics, U Penn, Philadelphia, PA, 5Department of Internal Medicine - Nephrology and Hypertension, U Cincinnati, Cincinnati, OH, 6Department of Medicine, JHU, Baltimore, MD, 7Internal Medicine - Gastroenterology, U Michigan, Ann Arbor, MI, 8Internal Medicine - Nephrology and Hypertension, Northwestern, Chicago, IL, 9Internal Medicine - Hepatology, Weill Cornell, NYC, NY, 10Internal Medicine - Gastroenterology, U Penn, Philadelphia, PA, 11Transplant Nephrology, U Penn, Philadelphia, PA
Meeting: 2020 American Transplant Congress
Abstract number: 281
Keywords: Donors, marginal, Hepatitis C, Kidney transplantation
Session Information
Session Name: All Organs: Viral Hepatitis
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: Kidneys from Hepatitis C virus (HCV)-infected deceased donors continue to be discarded at high rates despite small, successful studies of HCV-viremic donors into uninfected recipients. We conducted a multicenter clinical trial at 7 centers to (1) establish safety and efficacy of pre-emptive G/P in HCV-negative subjects receiving HCV-viremic kidney transplants (KT) and (2) describe outcomes of eligible patients not undergoing an HCV-viremic KT.
*Methods: Study candidates underwent education and informed consent. Donors were HCV-RNA positive, any genotype, with KDPI<85%. G/P was started post-transplant day 3 and continued for 8 weeks. The primary outcome was sustained virologic response at 12 weeks (SVR12) after finishing G/P.
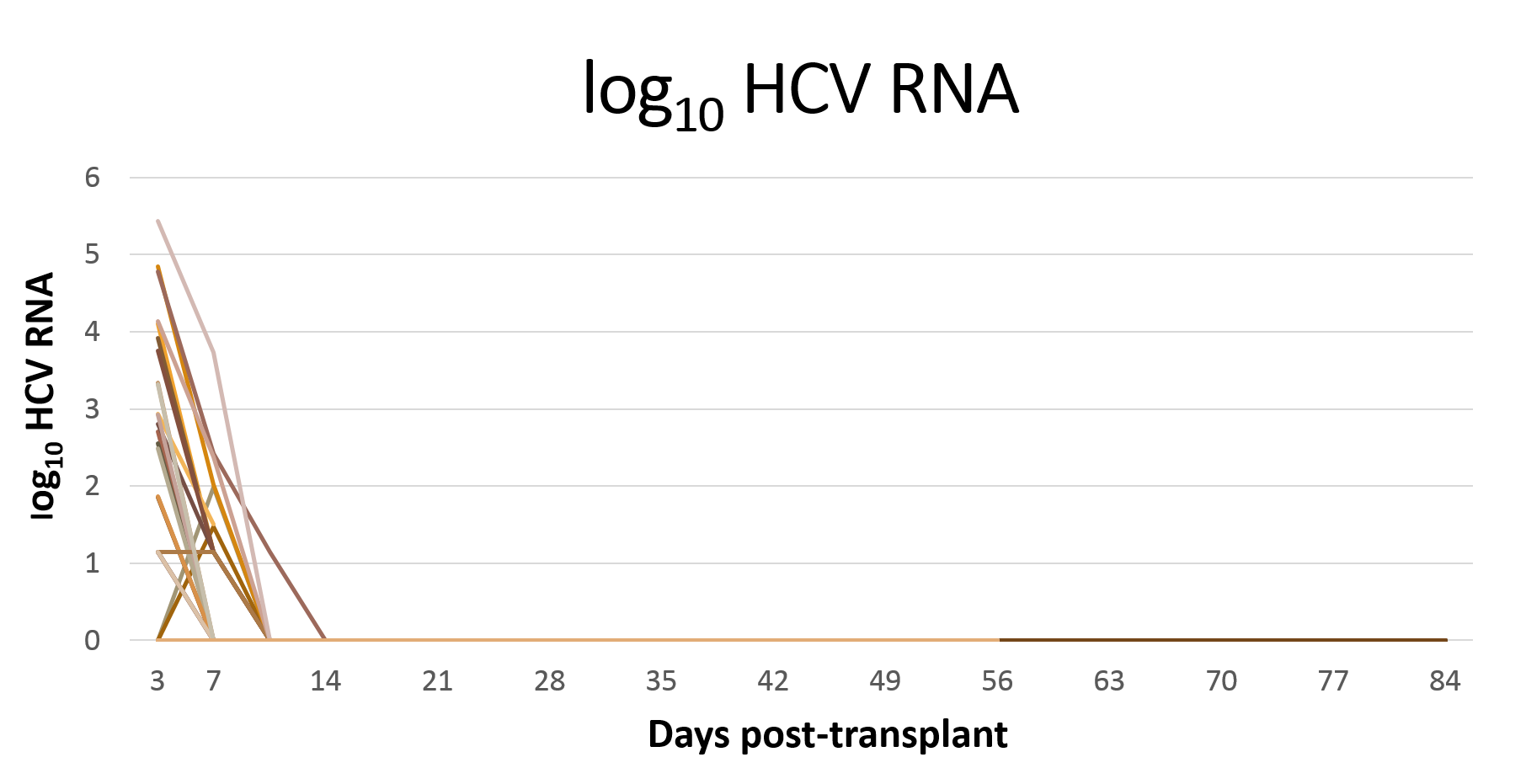
*Results: 76 patients were consented, 12 were excluded. Of 64 eligible patients, 30 underwent KT from HCV-viremic donors after a median 44 days (IQR 13-71). Mean recipient age was 55 (SD 8), 70% were male, 63% were Caucasian. Median waiting time at consent time was 959 days (IQR 666-1533). Donor KDPI was median of 53% (IQR 41-65). To date, all nine recipients with adequate follow-up have achieved SVR12; the remaining 21 participants have undetectable or rapidly declining HCV RNA (Figure 1), and all have improving creatinine. There were no G/P-related severe adverse events, including no instances of clinical liver dysfunction, acute rejection, or clinically significant CMV. All enrolled patients will be followed for 1-year post-KT or 1-year post enrollment, with all SVR12 results available by April 2020.
*Conclusions: Preliminary findings from the first multicenter standardized trial of pre-emptive G/P after KT from HCV-viremic kidneys into HCV-negative recipients suggest that this approach is highly effective, safe, with excellent allograft function and shortened waitlist time to transplant.
To cite this abstract in AMA style:
Sise M, Goldberg D, Kort J, Schaubel D, Alloway R, Durand C, Fontana R, Friedewald J, Brown R, Prenner S, Landis R, Reese P, Chung R. Multi-Center, Open-Label Trial of Pre-Emptive Glecaprevir/Pibrentasvir (G/P) to Treat Recipients of Transplanted Kidneys from Deceased Donors with Hepatitis C Virus (MYTHIC) [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/multi-center-open-label-trial-of-pre-emptive-glecaprevir-pibrentasvir-g-p-to-treat-recipients-of-transplanted-kidneys-from-deceased-donors-with-hepatitis-c-virus-mythic/. Accessed February 18, 2026.« Back to 2020 American Transplant Congress