Monitoring Efficacy of Tocilizumab Treatment for Chronic Antibody Mediated Rejection (cAMR) in Kidney Transplants Using Donor-Derived Cell-Free DNA (dd-cf-DNA)
1Comprehensive Transplant Center, Cedars Sinai Medical Ctr, Los Angeles, CA, 2HLA Laboratory, Cedars Sinai Medical Ctr, Los Angeles, CA
Meeting: 2022 American Transplant Congress
Abstract number: 1409
Keywords: Antibodies, Kidney transplantation, Outcome, Rejection
Topic: Clinical Science » Kidney » 45 - Kidney Chronic Antibody Mediated Rejection
Session Information
Session Name: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: IL-6/IL-6R have emerged as important targets for treating cAMR. Recent data show that long-term treatment can stabilize eGFR, reduce DSAs and result in a reduction of pathologic and molecular markers of immune injury in allograft biopsies. dd-cf-DNA has emerged as an important biomarker for diagnosing AMR/cAMR, however, the utility in predicting response to immunomodulatory therapies is lacking. Having non-invasive biomarkers to monitor therapeutic efficacy would be beneficial and reduce the need for biopsies. Here we examined the utility of dd-cf-DNA in patients receiving long-term treatment of cAMR with tocilizumab (TCZ) (anti-IL-6R).
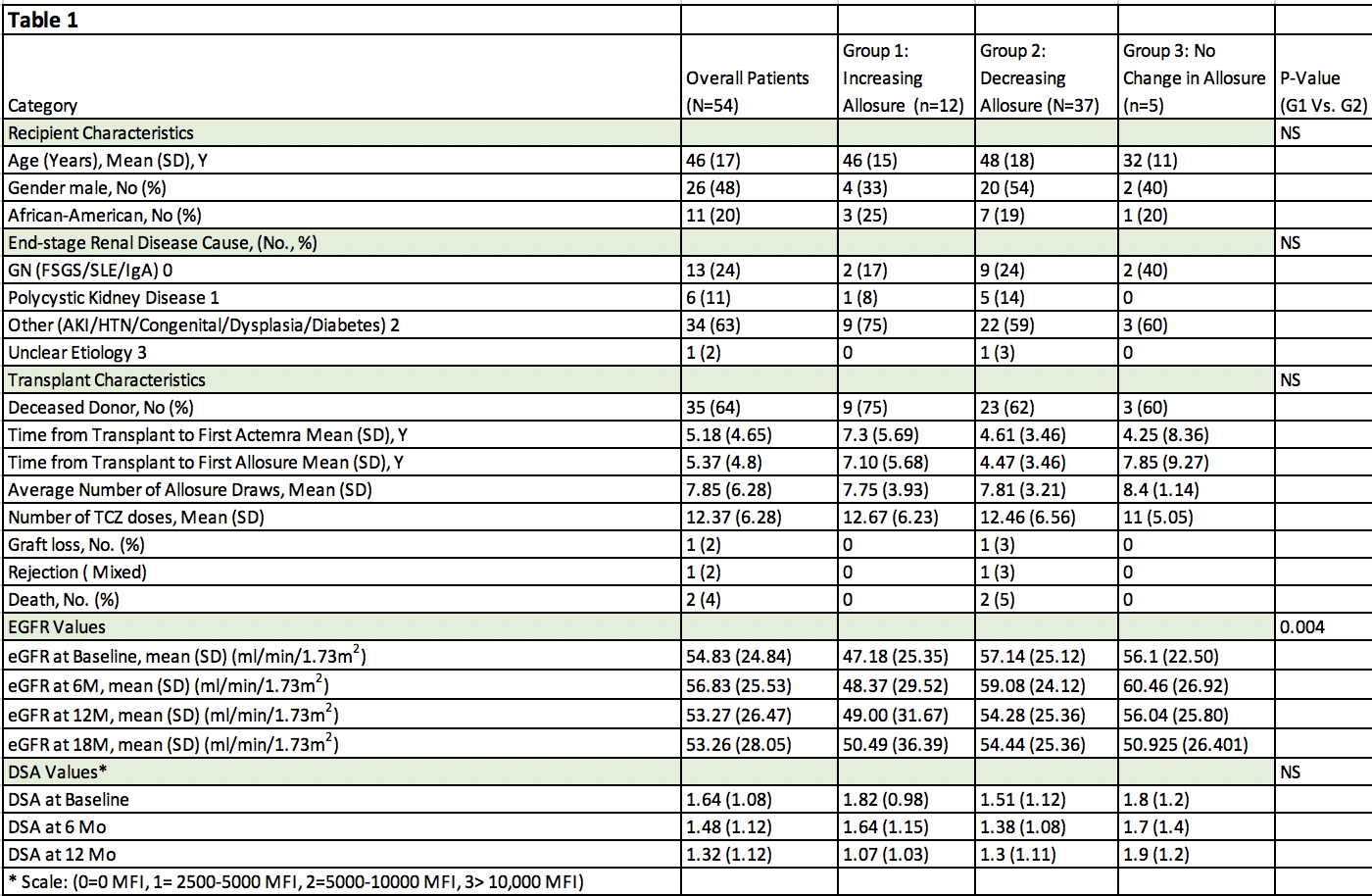
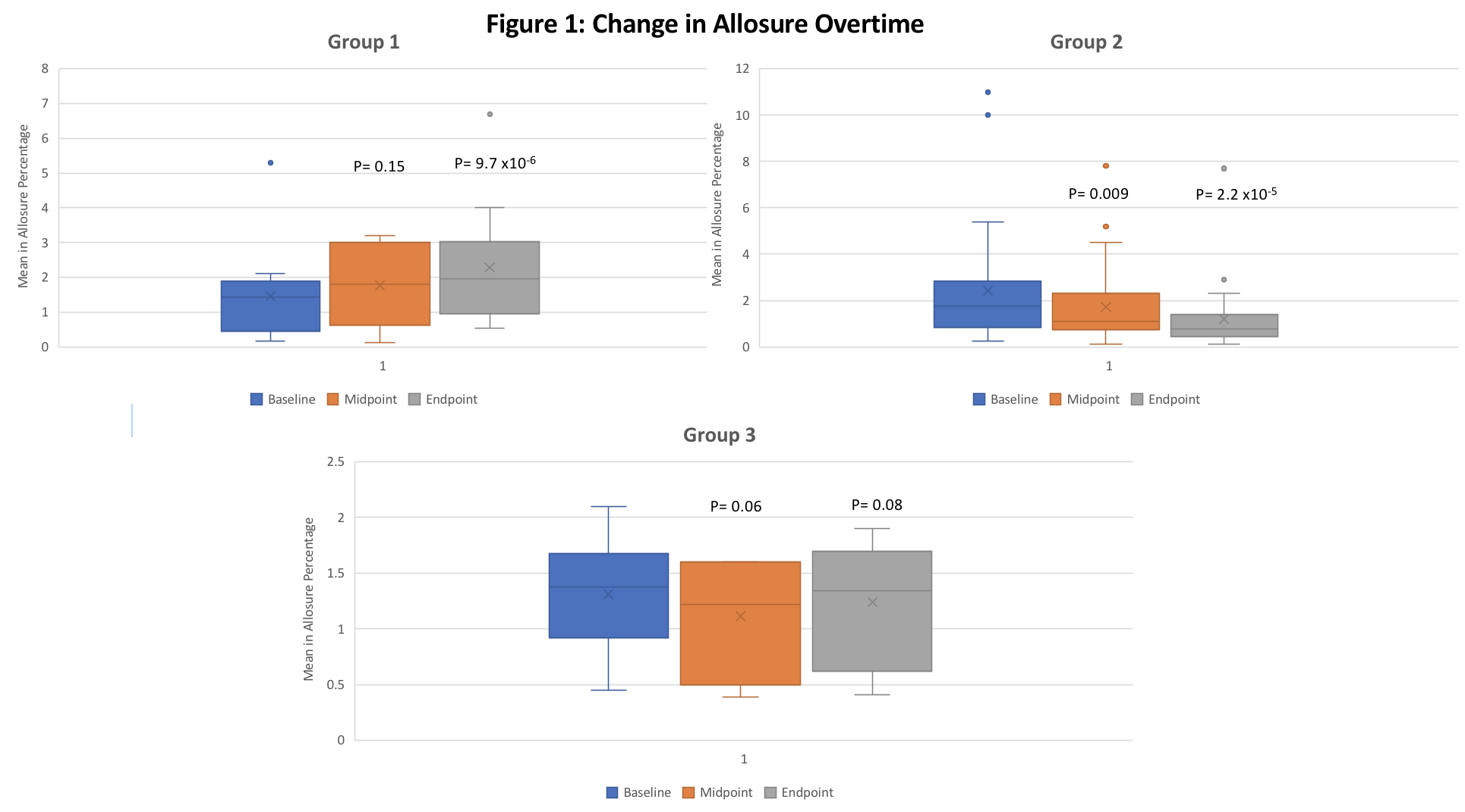
*Methods: 54 patients with biopsy-proven cAMR and DSA+ were treated with TCZ (8mg/kg/iv) monthly X12. Patients were also monitored at time 0, 6M & 12M for eGFR, reductions in DSA and % dd-cf-DNA (Allosure®). Patients were divided into 3 groups (G1: showing increasing dd-cf-DNA; G2: decreasing dd-cf-DNA and G3: stable dd-cf-DNA). Graft loss and deaths were analyzed. Assessment of AEs/SAEs was done.
*Results: Of 54 TCZ treated patients, 37 (68.5%) (G2) showed significant declines in dd-cf-DNA over 1 year of TCZ treatment, 12 (22%) (G1) showed increases and 5 (9.5%) (G3) showed no change (Table 1, Figure 1). DSA levels tended down in all groups. Importantly, eGFRs in G1 were significantly lower than G2 (p=0.004). 1 graft loss and 2 deaths occurred in G2. Overall, TCZ was well tolerated with good safety profile in most patients. Patients in whom TCZ was discontinued for ≥2M tended to show increases in dd-cf-DNA suggesting increasing allograft injury.
*Conclusions: dd-cf-DNA appears promising as a biomarker relating well to predicting responses to anti-IL-6R treatment in cAMR patients. 68.5% showed declining dd-cf-DNA that associated with significant improvements in eGFR. This data also suggests that for patients in G1 & G3 increasing or stable dd-cf-DNA may represent ongoing or TCZ-resistant rejection that could require intervention with other immunomodulatory agents.
To cite this abstract in AMA style:
Tang J, Vo A, Huang E, Zhang X, Ammerman N, Peng A, Najjar R, Sethi S, Lim K, Gillespie M, Badash N, Jordan S. Monitoring Efficacy of Tocilizumab Treatment for Chronic Antibody Mediated Rejection (cAMR) in Kidney Transplants Using Donor-Derived Cell-Free DNA (dd-cf-DNA) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/monitoring-efficacy-of-tocilizumab-treatment-for-chronic-antibody-mediated-rejection-camr-in-kidney-transplants-using-donor-derived-cell-free-dna-dd-cf-dna/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress