Molecular ABO Typing: An Effective Tool for Resolving Deceased Donor Indeterminate ABO Types
1Eurofins DPT, Los Angeles, CA, 2Eurofins DPT, Centennial, CO, 3Eurofins DPT, Centennial, CA, 4Eurofins DPT, denver, CO
Meeting: 2022 American Transplant Congress
Abstract number: 930
Keywords: Blood transfusion, Organ Selection/Allocation, Polymerase chain reaction (PCR), Screening
Topic: Basic Science » Basic Science » 11 - Histocompatibility and Immunogenetics
Session Information
Session Name: Histocompatibility and Immunogenetics
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: In order to evaluate molecular ABO typing as an alternative method of blood typing that may be employed when indeterminate serological typing results are obtained, we performed a retrospective analysis on ABO test orders for which serological typing did not produce a valid result.
*Methods: Data was reviewed from September 2020 to November 2021. Deceased donors were routinely ABO typed serologically using ID-MTS Gel Test (Ortho), IH-Reader 24 Gel System (Bio-Rad), or IH-1000 Gel System (Bio-Rad). For molecular testing, real time PCR was employed using LinkSēq ABO Typing Kits (One Lambda). Molecular typing was performed at the request of OPOs, as this test is not automatically ordered for all donors when a serological result cannot be obtained.
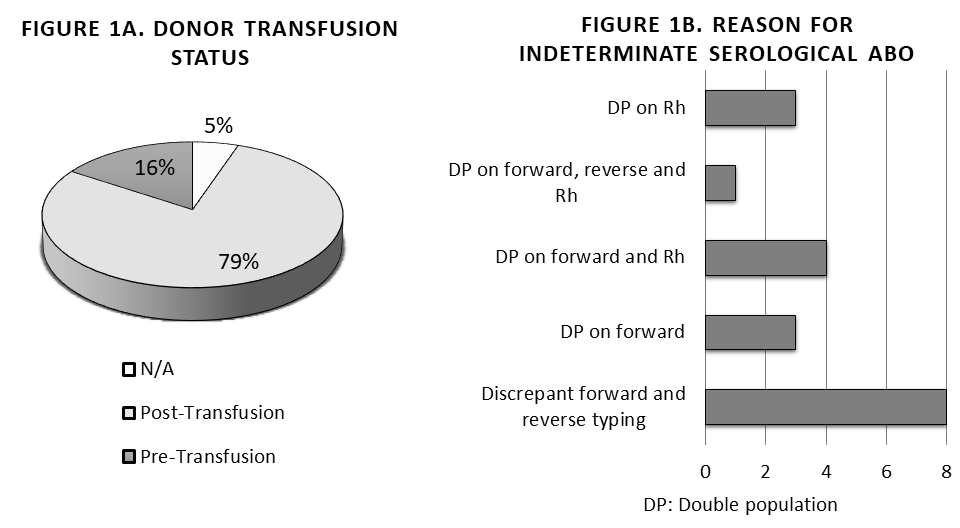
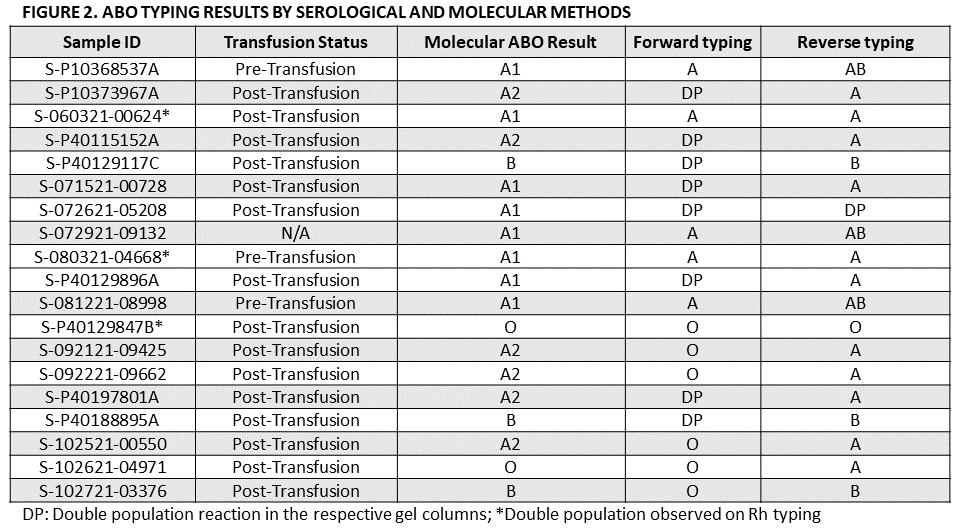
*Results: Over the period of our analysis, there were 19 donors for which serological ABO typing results could not be obtained. Figure 1A provides the transfusion status of the donors at the time of blood collection. 79% of samples were collected post-transfusion. Figure 1B lists the reasons why ABO typing could not be determined serologically. Molecular ABO results were successfully obtained using real-time PCR for all 19 donors. Figure 2 lists the results obtained using both test methods.
*Conclusions: ABO blood group compatibility between donor and recipient is one of the most important criteria of solid organ allocation. There are instances in which serological typing methods cannot determine a person’s ABO type. In June 2020, the OPTN issued a guidance document describing the use of molecular methods to aid in the determination of ABO typing. This was followed by a policy update in September 2020 requiring OPOs to include in their written protocols a process for addressing indeterminate blood type results. Molecular ABO typing is a rapid, viable method to help elucidate donor typing. The LinkSēq ABO real-time PCR assay takes 90 minutes and utilizes the same technology as HLA typing. The option for an alternative method for ABO determination in HLA laboratories should be considered as an aid to increase the number of deceased donors that can be allocated to our nation’s Waitlist.
To cite this abstract in AMA style:
Tanglao D, Musa SS, Chew JJ, Santiago L, Herron K, O'Neale B, Lemp NA, Dionne SO. Molecular ABO Typing: An Effective Tool for Resolving Deceased Donor Indeterminate ABO Types [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/molecular-abo-typing-an-effective-tool-for-resolving-deceased-donor-indeterminate-abo-types/. Accessed February 16, 2026.« Back to 2022 American Transplant Congress