Minimally Invasive Panel (MIP) as an Alternative for Surveillance Biopsies, A
Nephrology/Internal Medicine, UC Davis Medical Center, Sacramento, CA
Surgery/Transplant, UC Davis Medical Center, Sacramento, CA
Meeting: 2013 American Transplant Congress
Abstract number: 296
Background: early post-transplant events such as subclinical rejections have a measurable impact on log-term graft survival, therefore making screening for such events with surveillance biopsies a key component of management of kidney transplant patients. However, searching for biomarkers with less invasive and costly methods would be desirable.
Methods: A minimally invasive panel (MIP) consisting of: donor specific antibodies (by solid-phase essay, cut-off 500 MFI), proteinuria (urine protein to creatinine ratio, cut-off 0.5), and serum creatinine (estimated-GFR by MDRD method) were correlated with biopsy findings and long-term graft survival.
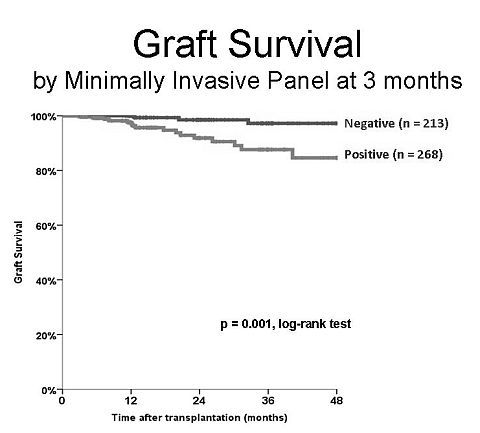
Results: Since 2007 481 patients underwent a 3-month surveillance biopsy and simultaneously a MIP was drawn. Ninety-six (15.4%) of biopsies showed no abnormalities and 213 pts (44 %) has a completely negative MIP. A negative MIP was associated with lower incidence of subclinical rejection (2.2%, missing 4 Banff borderline acute rejections), glomerular abnormalities (5.9%, missing 4 cases of IgA, 1 of each: membranous, MPGN2, and diabetes nephropathy, plus 4 cases of mesangium expansion with and without deposits), and 3 cases of chronic allograft injury (IFTA > 25%). Graft survival for the group with negative MIP was 97% at 3 years, significantly better than patients with positive MIP (85%, p=0.001, log-rank test) figure 1. At the same time a biopsy without abnormalities was associated with a graft survival of 94% at 3yr (88% for pts with an abnormal biopsy (88%, p=0.001). Sensitivity, specificity and negative predictive value for 3years graft failure was 83, 56,95% for MIP and 35, 93, and 95% for 3 months bx.
Conclusions: A minimally invasive panel drawn at 3 months post transplant effectively identify a group of recipients at a low risk for long-term graft failure. As a screaning tool, MIP was better than 3 months bx. We speculate that screening recipients with this panel could have spared 44% of patients from a surveillance biopsy, without compromising outcomes.
To cite this abstract in AMA style:
Alnimri M, Adey D, Gallay B, Perez R, Mattos ADe. Minimally Invasive Panel (MIP) as an Alternative for Surveillance Biopsies, A [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/minimally-invasive-panel-mip-as-an-alternative-for-surveillance-biopsies-a/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
