Longitudinal Studies of Blood Anellovirus DNA Prior to Acute Rejection or Major Infection Events in Pediatric Kidney Transplant Recipients (PKTx)
Washington University in St Louis, St. Louis, MO
Meeting: 2021 American Transplant Congress
Abstract number: 1007
Keywords: Infection, Kidney transplantation, Pediatric, Rejection
Topic: Clinical Science » Kidney » Kidney: Pediatrics
Session Information
Session Name: Kidney: Pediatrics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Blood levels of the ubiquitous but non-pathogenic anellovirus (AnV) taxa have been postulated to represent a biomarker of the overall state of immunosuppression after transplantation. Prior studies, largely cross-sectional in adults, suggest that levels of the AnV rise with major infection events (MIE) and drop with acute rejection (AR).
*Methods: We have performed longitudinal biobanking of serum in our PKTx population since 2013, combined with monthly testing for opportunistic viruses DNA by PCR from months 1-12 post-transplant and recording of clinical events. In this study we assayed longitudinal biobanked and prospective serum samples for AnV DNA levels (copies/mL) and associated to standard of care monitoring and clinical results. To adjust for repeated measurements within subjects, we used a linear mixed model approach to test for differences in AnV DNA levels between complication groups (None, MIE at time of sample, AR at time of sample). A log transformation was used to better meet model assumptions, and results are reported on the log scale.
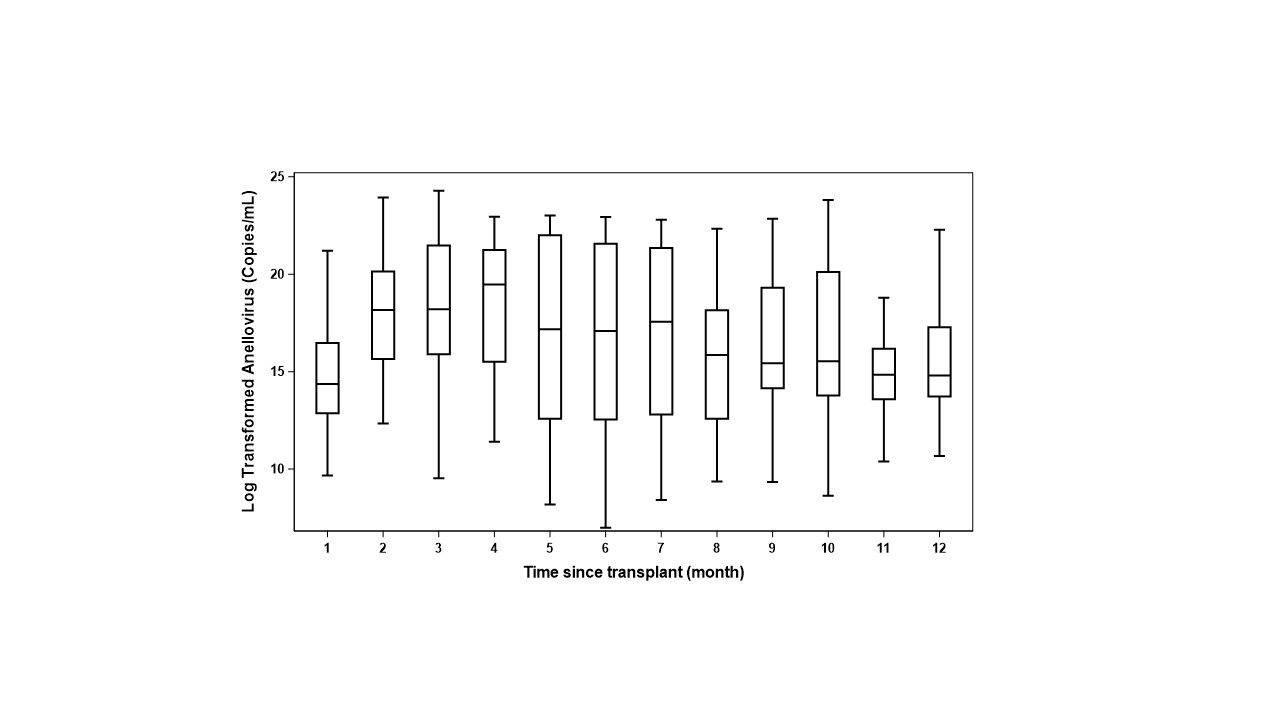
*Results: In 271 plasma samples from 46 children (Males 52%, white 83%, deceased donor 69%), as shown in Figure, most serum samples are positive for AnV through all time points. Further, box plots of longitudinal results in Figure show a slight increase in median AnV DNA levels at 2-4 months, the peak time period for opportunistic viral replication. Mixed model results showed that the log AnV DNA (copies/mL) was elevated by 0.5 log for the MIE group (adjusted mean of 17.3 [95% CI, 16.1 to 18.4]) compared to the no complications group (adjusted mean of 16.8 [95% CI, 15.9 to 17.7]) (P=0.41). In contrast, results for the AR group showed a lowering of log AnV DNA (copies/mL) (14.3 [95% CI, 15.9 to 17.7]) by 2.5 log compared to the no complications group (P=0.12). Although neither result was statistically significant (likely due to small sample size), both results are consistent with the concept from cross-sectional studies of AnV load as an immunosuppression state biomarker.
*Conclusions: In our PKTx cohort, our results suggest that longitudinal AnV levels that account for within patient variability also seem to associate to AR and MIE events. Further prospective longitudinal testing in a larger multicenter cohort is recommended.
To cite this abstract in AMA style:
Dandamudi R, Gu H, Goss C, Gula H, Wylie K, Dharnidharka V. Longitudinal Studies of Blood Anellovirus DNA Prior to Acute Rejection or Major Infection Events in Pediatric Kidney Transplant Recipients (PKTx) [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/longitudinal-studies-of-blood-anellovirus-dna-prior-to-acute-rejection-or-major-infection-events-in-pediatric-kidney-transplant-recipients-pktx/. Accessed March 9, 2026.« Back to 2021 American Transplant Congress