Longer-Term Outcomes for Hepatitis C Virus Negative Recipients of Kidneys from Hepatitis C Virus Nucleic Acid Test Positive Donors: The THINKER Trial
1University of Penn, Philadelphia, PA, 2Gift of Life, Philadelphia, PA
Meeting: 2019 American Transplant Congress
Abstract number: 17
Keywords: Donation, Donors, marginal, Hepatitis C, Kidney transplantation
Session Information
Session Name: Concurrent Session: Kidney Deceased Donor Allocation I
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:30pm-3:42pm
Presentation Time: 3:30pm-3:42pm
Location: Ballroom B
*Purpose: Two trials of transplanting hepatitis C virus (HCV) nucleic acid test (NAT) positive kidneys into HCV-negative recipients have been published, both with 100% HCV cure rates but small numbers and short term results. We now report antiviral response and allograft outcomes for 45 kidney transplant recipients (KTR) from the THINKER trial with data up to 24 months post-transplant. Additionally, we compared HCV treatment responses between 7 THINKER KTRs and 5 HCV-negative heart transplant recipients (HTR) from the same donors enrolled in the USHER trial.
*Methods: HCV-negative patients aged 40-65 years on the waitlist for kidney transplant were included in THINKER. Donor HCV genotyping allowed us to restrict transplant only to kidneys from GT 1 or 4 donors, with grazoprevir/elbasvir treatment on day 3 post-transplant when recipient HCV was detected.
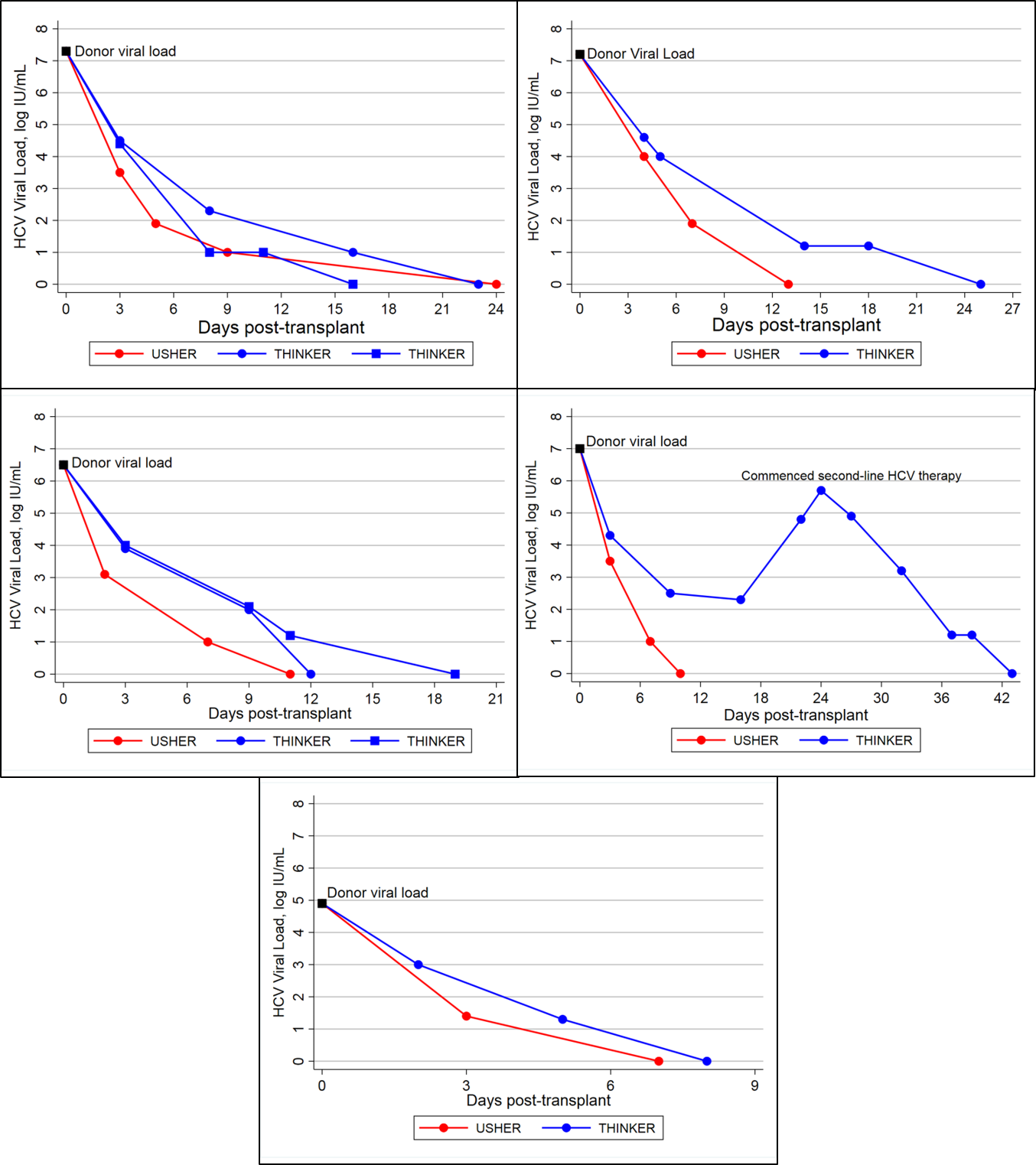
*Results: From 01/16-11/18, 45 patients underwent kidney transplant with HCV-NAT+ kidneys (median KDPI: 48%, IQR: 35-58%); 40 (88.9%) were genotype 1a, 4 (8.9%) genotype 1b, and 1 (2.2%) genotype 4. All 45 patients were treated with thymoglobulin, and 43 (95.6%) had detectable HCV RNA on post-op day 3. An additional two patients did not become viremic until post-transplant day 5. As of 11/25/18, 39 have been cured (defined as sustained virologic response by 12-weeks [SVR-12]), while the other 6 are completing treatment or in follow-up. One kidney recipient, however, initially responded to antiviral therapy but had rising HCV viral load by day 18 and was given zepatier + sofosbuvir + ribavirin for 16 weeks; on this regimen, the patient reached SVR-12. By contrast, the HTR from the same donor rapidly responded to grazoprevir/elbasvir without any additional therapy. The Figure shows HCV NAT over time for this KTR and HTR (panel 4) and recipients from 4 other shared donors.
In THINKER, there have been no deaths, allograft failures or rejection episodes. For the 10 THINKER subjects who have reached 24 months, the median creatinine was 1.07 mg/dL (IQR: 1.0, 1.28).
*Conclusions: We demonstrate longer-term efficacy of transplanting kidneys from HCV-infected donors into HCV-negative patients. Wider use of HCV-infected organs should be a priority. However, for centers developing their own programs, our case of early antiviral therapy failure indicates the importance of closely monitoring HCV NAT after transplant and having a pathway for patients to receive second-round therapy.
To cite this abstract in AMA style:
Reese P, Abt P, Blumberg E, Bloom R, Sawinski D, Porrett P, Woodards A, Reddy K, Deerlin Vvan, Hasz R, Levine M, McLean R, Sicilia A, Goldberg D. Longer-Term Outcomes for Hepatitis C Virus Negative Recipients of Kidneys from Hepatitis C Virus Nucleic Acid Test Positive Donors: The THINKER Trial [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/longer-term-outcomes-for-hepatitis-c-virus-negative-recipients-of-kidneys-from-hepatitis-c-virus-nucleic-acid-test-positive-donors-the-thinker-trial/. Accessed March 11, 2026.« Back to 2019 American Transplant Congress