Long-Term Outcomes in Epstein-Barr Virus (EBV)-Positive Patients Receiving Belatacept (Bela) or Cyclosporine (CsA) in BENEFIT-EXT.
S. Florman,1 J. Medina Pestana,2 M. del C Rial,3 L. Rostaing,4 D. Kuypers,5 A. Matas,6 T. Wekerle,7 J. Grinyó,8 U. Meier-Kriesche,9 M. Polinsky,9 H. Zhao,9 A. Durrbach.10
1Mount Sinai Med Ctr, New York
2Hosp do Rim, Sao Paulo, Brazil
3Instituto de Nefrologia, Buenos Aires, Argentina
4Univ Hosp, Toulouse, France
5Univ Hosp Leuven, Leuven, Belgium
6Univ of Minnesota, Minneaopolis
7Med Univ of Vienna, Vienna, Austria
8Univ Hosp Bellvitge, Barcelona, Spain
9BMS, Lawrenceville
10Univ Hôpital of Bicêtre, Le Kremlin-Bicêtre, France.
Meeting: 2016 American Transplant Congress
Abstract number: D138
Keywords: Cadaveric organs, Epstein-Barr virus (EBV), Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
At 7 yrs post transplant in BENEFIT-EXT (NCT00114777), bela-treated pts had similar graft survival and improved renal function vs CsA-treated pts. Bela is indicated for use only in EBV-positive pts. Outcomes in BENEFIT-EXT pts who were EBV-positive prior to transplant are described.
Recipients of extended criteria donor kidneys were randomized to receive bela more intense (MI), bela less intense (LI), or CsA immunosuppression. All randomized, transplanted EBV-positive pts were analyzed through 7 yrs post-transplant. Time to death or graft loss was compared between regimens using Cox regression. GFR was estimated from months 1–84 using a repeated measures model.
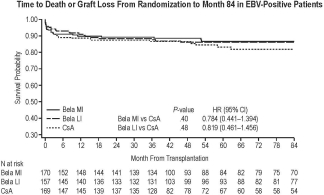
Of 543 randomized pts, 496 were EBV-positive. Of these, 121/170 bela MI-treated, 124/157bela LI-treated, and 102/169 CsA-treated pts were evaluable for death/graft loss at 7 yrs. Hazard ratios comparing time to death/graft loss were 0.784 for bela MI vs CsA (P=.40) and 0.819 for bela LI vs CsA (P=.48) (Fig.). Serious AE rates were similar (87%, bela MI; 89%, bela LI; 84%, CsA). Estimated mean GFR increased over 7 yrs for both bela regimens but declined for CsA (estimated mean GFR at yr 7: bela MI, 53.5; bela LI, 53.6; CsA, 36.1 mL/min/1.73 m2). GFR slopes diverged between bela and CsA over time; the interaction of the treatment vs time effect deriving from the model favored each bela regimen vs CsA (P≤.001). Among bela-treated pts, PTLD occurred in 3 EBV-positive and 5 EBV-negative pts by month 84.
Outcomes in the subset of EBV-positive pts were consistent with those observed in the ITT population: bela was associated with similar death/graft loss and improved renal function vs CsA.
CITATION INFORMATION: Florman S, Medina Pestana J, del C Rial M, Rostaing L, Kuypers D, Matas A, Wekerle T, Grinyó J, Meier-Kriesche U, Polinsky M, Zhao H, Durrbach A. Long-Term Outcomes in Epstein-Barr Virus (EBV)-Positive Patients Receiving Belatacept (Bela) or Cyclosporine (CsA) in BENEFIT-EXT. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Florman S, Pestana JMedina, Rial MdelC, Rostaing L, Kuypers D, Matas A, Wekerle T, Grinyó J, Meier-Kriesche U, Polinsky M, Zhao H, Durrbach A. Long-Term Outcomes in Epstein-Barr Virus (EBV)-Positive Patients Receiving Belatacept (Bela) or Cyclosporine (CsA) in BENEFIT-EXT. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-outcomes-in-epstein-barr-virus-ebv-positive-patients-receiving-belatacept-bela-or-cyclosporine-csa-in-benefit-ext/. Accessed February 26, 2026.« Back to 2016 American Transplant Congress
