Long-Term Calcineurin Inhibitor (CNI) and Corticosteroid (CS) Avoidance Using Belatacept: The CTOT-10 Experience
Emory Transplant Center
Univ of Alabama-Birmingham
UC-San Francisco
NIAID/NIH
Rho
Meeting: 2013 American Transplant Congress
Abstract number: 15
Belatacept is approved a component of a CNI-free immunosuppressive regimen in EBV + adults undergoing renal transplantation. The intent of this NIH-sponsored CTOT study was to compare two different approaches to achieve maintenance immunosuppression with belatatcept and MMF to a regimen consisting of tacrolimus (tac) and MMF.
19 primary renal transplant recipients who had immunity to EBV, a negative cross-match, and a PRA < 30% were randomized to 1 of 3 arms (Figure). All patients groups received perioperative steroids and maintenance MMF. Patients in groups 1 and 2 were induced with alemtuzumab and maintained on tac or belatacept respectively. Patients in group 3 were induced with basiliximab and 3 months of tac and maintained on belatacept.
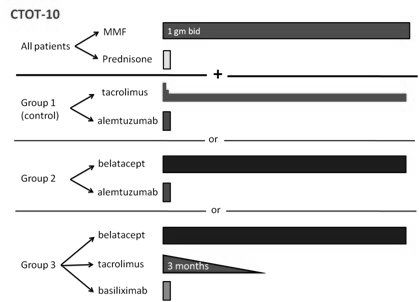
Short-term results are shown in Table 1.
| Functioning grafts | |||||||
| Group | Vascular thrombosis | Graft Loss | Patient Death | Rejection (initial Banff score) | Creatinine(mg/dl) | eGFR (ml/min/1.73sq.m) | Mean follow up (weeks) |
| 1 (N=6) | 1 | 0 | 1 | 0 | 1.5 | 49 | 47 |
| 2 (N=6) | 3 | 3 | 0 | 2 (1A, 2B) | 1.8 | 46 | 32 |
| 3 (N=7) | 0 | 0 | 0 | 3 (2A, 2A, 2B) | 1.5 | 52 | 42 |
There was one late death from endocarditis and sepsis (Group 1). All patients who experienced rejection had a return to baseline Cr following treatment. Three of 4 episodes of rejection in group 3 occurred shortly after discontinuation of tac. The baseline Cr of patients with functioning grafts indicated excellent renal function. Clinical monitoring detected 1, 1, and 2 cases of BK viremia without renal dysfunction in the respective groups. One patient in group 3 had transient, asymptomatic CMV viremia. Due to safety concerns, the protocol was halted.
Findings from this small cohort include a high incidence of vascular events and rejection. All thromboses occurred in patients receiving alemtuzumab and belatacept raising the possibility of an association. The high incidence of rejection in patients receiving belatacept suggests that these regimens may not be sufficiently immunosuppressive. The CTOT consortium is exploring alternative protocols using belatacept in renal transplantation.
To cite this abstract in AMA style:
Newell K, Mannon R, Mehta A, Tomlanovich S, Morrison Y, Ikle D, Goodman J, Mehta S, Bridges N, Stock P, Larsen C. Long-Term Calcineurin Inhibitor (CNI) and Corticosteroid (CS) Avoidance Using Belatacept: The CTOT-10 Experience [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/long-term-calcineurin-inhibitor-cni-and-corticosteroid-cs-avoidance-using-belatacept-the-ctot-10-experience/. Accessed March 3, 2026.« Back to 2013 American Transplant Congress
