Living Kidney Donor Pharmaceutical Care Needs Identified Through Linked Transplant Registry and Pharmacy Claims Data.
1St Louis Univ, St Louis, MO
2SRTR, Minneapolis, MN
3Symphony Health, Philadelphia, PA
4Johns Hopkins, Baltimore, MD
5Western Univ, London, Canada
6Univ Alberta, Edmonton, AB, Canada
7E Carolina Univ, Greenville, NC
8Washington Univ, St. Louis, MO
Meeting: 2017 American Transplant Congress
Abstract number: 377
Keywords: Donation, Kidney, Outcome, Post-operative complications
Session Information
Session Name: Concurrent Session: Logistic and Programatic Challenges in Kidney Living Donation
Session Type: Concurrent Session
Date: Monday, May 1, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: E450b
Limited data are available on pharmaceutical care needs of living kidney donors (LKD).
We integrated 1) national US Scientific Registry of Transplant Recipients data for LKD (1987-2012) with 2) pharmacy fill records from a nationwide pharmacy claims warehouse to examine utilization patterns of diabetes treatments, antihypertensive medications, and antidepressants as measures of these conditions before and after donation.
The linked data captured 32,065 LKD actively filling in the first year before, and 36,597 actively filling in the first year after donation (Table 1). A very small but surprising fraction of LKD were treated with insulin or an oral hypoglycemic agent in the 3 years prior to donation. In the first decade following donation, 0.4% received insulin and 2.3% received an oral glucose-lowering agent. Angiotensin converting enzyme inhibitors comprised the most common class of antihypertensive agent used in the years after donation, with use in 11.3% by year 10. Among the sample, 8.6% received a diuretic by year 10. The most common antidepressant prescriptions filled before and after donation were selective serotonin reuptake inhibitors (SSRI). 9.3% of LKD filled an SSRI prescription in the year before donation, and 8.7% in the year after donation.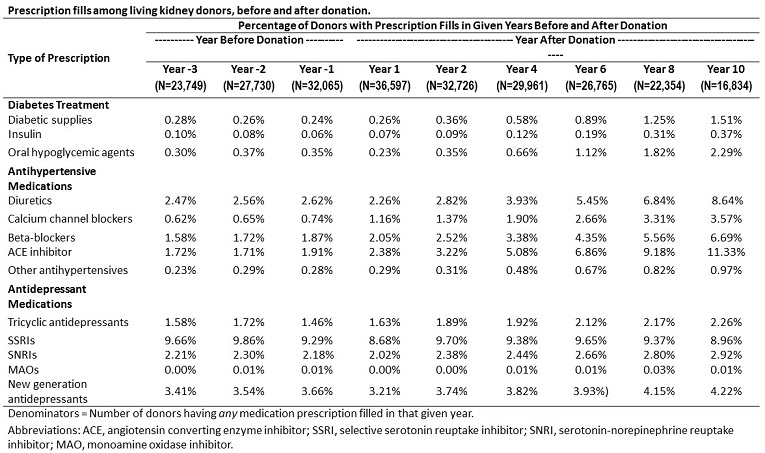 Use of antihypertensive and diabetes medications increase over time after live kidney donation, while antidepressant use appears stable. These data demonstrate the rich potential for linked transplant registry data and pharmacy claims data to examine the incidence and prevalence of medically-treated conditions before and after donation. Future work should compare pharmaceutical care needs of living donors to that of controls with similar baseline good health.
Use of antihypertensive and diabetes medications increase over time after live kidney donation, while antidepressant use appears stable. These data demonstrate the rich potential for linked transplant registry data and pharmacy claims data to examine the incidence and prevalence of medically-treated conditions before and after donation. Future work should compare pharmaceutical care needs of living donors to that of controls with similar baseline good health.
CITATION INFORMATION: Lentine K, Gustafson S, Schnitzler M, Hess G, Segev D, Garg A, Lam N, Axelrod D, Brennan D, Randall H, Kasiske B. Living Kidney Donor Pharmaceutical Care Needs Identified Through Linked Transplant Registry and Pharmacy Claims Data. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Lentine K, Gustafson S, Schnitzler M, Hess G, Segev D, Garg A, Lam N, Axelrod D, Brennan D, Randall H, Kasiske B. Living Kidney Donor Pharmaceutical Care Needs Identified Through Linked Transplant Registry and Pharmacy Claims Data. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/living-kidney-donor-pharmaceutical-care-needs-identified-through-linked-transplant-registry-and-pharmacy-claims-data/. Accessed March 8, 2026.« Back to 2017 American Transplant Congress
