Likelihood of Improving or Maintaining Renal Function over Five Years with Belatacept or CsA: Insights from the BENEFIT Long-Term Extension Study
Univ Hosp Bellvitge, Spain
Bicetre Hosp, France
Univ Hospital, France
Med College of Wisconsin, WI
Vanderbilt Univ, TN
Baylor Univ Med Ctr, TX
UCSF Kidney Transpl Svc, CA
BMS, NJ
Hosp do Rim e Hipertensao, Brazil
Meeting: 2013 American Transplant Congress
Abstract number: 492
Introduction: In results reported from BENEFIT, the renal function benefit observed in belatacept (bela)-treated pts in the early post-transplant period was maintained over time with a consistent safety profile. Changes in cGFR stage over 5 yrs in the long-term extension (LTE) cohort are reported here.
Methods: In BENEFIT, recipients of living or standard-criteria deceased donor kidneys were randomized to a more or less intensive (LI) regimen of bela or CsA. Pts remaining on assigned therapy after 3 yrs were eligible to enter the LTE. This posthoc analysis assessed shifts in cGFR stage between Months (Mos) 12 & 60 in the LTE population & focuses on the approved LI regimen. cGFR stages were defined per the KDOQI classification of CKD stages. cGFR was imputed as 0 for death or graft loss. Among pts enrolled in the LTE, 139/165 LI & 102/136 CsA pts had cGFR data available at both Mos 12 & 60.
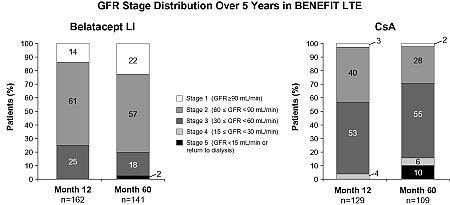
Results: GFR stage at Mos 12 & 60 in LI & CsA pts of the LTE cohort are shown in the Figure. Among pts in Stage 1 at Mo 12, 79% (15/19) LI & 67% (2/3) CsA pts maintained their GFR stage through Mo 60. Among pts in Stage 2 at Mo 12, 91% (77/85) LI & 49% (21/43) CsA pts maintained or improved their GFR stage through Mo 60. In Stage 3 pts, GFR stage was maintained or improved in 94% (33/35) LI & 77% (40/52) CsA pts from Mo 12–60. There were no LI pts who were in Stage 4 at Mo 12; all CsA pts (4/4) in Stage 4 at Mo 12 maintained or improved their GFR stage through Mo 60. There were no LI or CsA pts who were in Stage 5 at Mo 12 in the LTE cohort.
Conclusions: In the BENEFIT LTE cohort at 5 yrs post-transplant, a higher percentage of belatacept pts were in Stages 1 & 2, and a higher percentage of CsA pts were in Stage 3. Belatacept pts were more likely than CsA pts to experience maintained or improved renal function over 5 yrs.
Durrbach, A.: Employee, BMS. Rostaing, L.: Grant/Research Support, Wyeth, Other, BMS, Participation in Advisory Board, Veloxis, Honorarium. Vincenti, F.: Grant/Research Support, Bristol-Myers Squibb. Pupim, L.: Employee, Bristol-Myers Squibb (Belatacept). Coffey, M.: Employee, Bristol-Myers Squibb. Pestana, J.: Grant/Research Support, BMS.
To cite this abstract in AMA style:
Grinyo J, Durrbach A, Rostaing L, Bresnahan B, Helderman J, Rice K, Vincenti F, Pupim L, Coffey M, Pestana J. Likelihood of Improving or Maintaining Renal Function over Five Years with Belatacept or CsA: Insights from the BENEFIT Long-Term Extension Study [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/likelihood-of-improving-or-maintaining-renal-function-over-five-years-with-belatacept-or-csa-insights-from-the-benefit-long-term-extension-study/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
