Large Academic Center Experience with Treatment of Suspected/Confirmed Kidney Transplant Active Antibody Mediated Rejection
1Transfusion Medicine Div, Univ of Pennsylvania, Philadelphia, PA, 2Pharmacy, Hosp of Univ of Pennsylvania, Philadelphia, PA, 3Renal Div, Univ of Pennsylvania, Philadelphia, PA, 4Pharmacy, Hospital of University of Pennsylvania, Philadelphia, PA
Meeting: 2020 American Transplant Congress
Abstract number: A-015
Keywords: Immunosuppression, Kidney transplantation, Rejection, Renal dysfunction
Session Information
Session Name: Poster Session A: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIG) are common active antibody mediated rejection (ABMR) treatments in kidney transplant recipients (KTRs), but with unclear benefits. The study purpose was to 1) describe diagnostic characteristics of KTRs undergoing treatment for suspected or confirmed active ABMR with TPE at our center and 2) to assess graft outcomes.
*Methods: We retrospectively reviewed HIV negative adult KTRs treated for suspected or confirmed active ABMR with TPE at least once at our center from 1/1/12-11/1/18. We reviewed biopsy reports and donor specific antibodies (DSAs) for all KTRs around time of treatment, and compared to active AMR Banff 2017 criteria. We evaluated DSAs at treatment initiation, and months 1,3,6,12,24 post-treatments, as well as graft survival data, collected until 10/1/19.
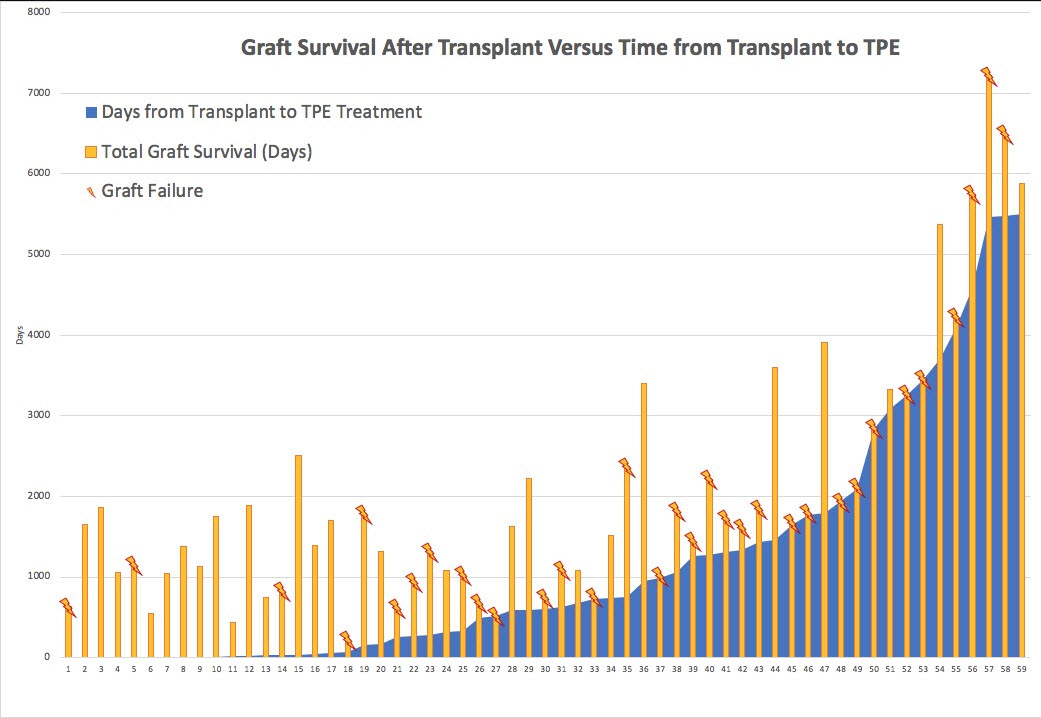
*Results: Fifty-nine KTRs received TPE and 96% received concurrent IVIG. By biopsy report review, less than half (43%) of KTRs treated for active ABMR met active ABMR Banff 2017 criteria, (19% had 1/3 criteria (DSA only), 15% had chronic ABMR, 14% met 2/3 active criteria, 5% met 0/3 active ABMR Banff criteria and 3% were indeterminate). Mean(SD) age of KTRs was 44 (±14) yrs, 35% were female, 51% were of black race and 19% were re-transplants. Of KTRs that met active ABMR Banff 2017 criteria, 42% had graft loss within 1 yr of treatment versus 18% who did not meet critera. [Table]. Of KTRs who had graft survival >3 yrs post-treatment only 27% met active ABMR Banff 2017 criteria. Only 23% of KTRs had graft loss <3 yrs post-transplant and 77% had graft survival for >3 yrs. Overall, 34% of KTRs had graft survival for >3 yrs post-treatment. [Figure].
*Conclusions: 1) Over 50% of KTRs who received active ABMR treatment (with at least one TPE), did not meet active ABMR Banff 2017 criteria. 2) Treated KTRs who meet active ABMR Banff 2017 criteria have significantly more graft loss compared to those who do not, suggesting criteria describe differences between these KTRs. 3) Studies are needed to define optimal interventions within populations.
| Graft failure at > 3 yrs or no failure (%) | Graft failure at <1 yr (%) | Graft failure between >1 yr < 3 yrs (%) | |
| 0/3 Criteria for active ABMR (n = 3) | 100 | 0 | 0 |
| 1/3 Criteria for active ABMR, (DSA only) (n = 11) | 73 | 18 | 9 |
| 2/3 Criteria for active ABMR (n = 8) | 63 | 25 | 13 |
| 3/3 Criteria for active ABMR (n = 26) | 31 | 42 | 27 |
| Chronic ABMR (n = 9) | 56 | 22 | 22 |
| Indeterminate findings (n = 2) | 100 | 0 | 0 |
To cite this abstract in AMA style:
Reece JT, Steiner BA, Bhoj V, Aqui N, Bloom RD, Trofe-Clark J. Large Academic Center Experience with Treatment of Suspected/Confirmed Kidney Transplant Active Antibody Mediated Rejection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/large-academic-center-experience-with-treatment-of-suspected-confirmed-kidney-transplant-active-antibody-mediated-rejection/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress