Kidney Transplant Induction Therapies: Risks of Death, Allograft Failure, Sepsis, and Cancer.
1University of Pennsylvania, Philadelphia
2The Children's Hospital of Philadelphia, Philadelphia
3Cleveland Clinic, Cleveland.
Meeting: 2016 American Transplant Congress
Abstract number: C35
Keywords: Kidney transplantation
Session Information
Session Name: Poster Session C: Clinical Science - Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Induction therapies are commonly used during kidney transplantation (~85% of transplants in 2012). Existing studies of induction therapy are limited by short follow-up and/or absence of information about associated complications including sepsis, lymphoma, and skin cancer. Using linked data from the United Network for Organ Sharing and Centers for Medicare and Medicaid Services, we compared outcomes between 3 induction therapies for recipients transplanted between 1/2003 through 12/2008. We matched recipients based on 15 clinical and demographic characteristics and created 1:1 alemtuzumab-thymoglobulin (6,840 pairs) and alemtuzumab-basiliximab (6,569 pairs) matches, enabling comparisons across all 3 therapies. Primary outcomes were death, death or allograft failure, death or sepsis, and death or lymphoma. Secondary outcomes were death or melanoma and death or non-melanoma skin cancer. Thymoglobulin patients had a lower rate of death or allograft failure relative to alemtuzumab patients (HR = 0.89, p < 0.01). Basiliximab patients had a higher rate of death or sepsis relative to alemtuzumab patients (HR = 1.12, p < 0.01), which may be explained by other factors (e.g., frailty, oral prednisone use) associated with typical alemtuzumab and basiliximab profiles. Differences for other outcomes, like death, were not statistically significant. Survival curves for all 3 therapies suggested outcome differences among induction therapies were not clinically important. Sub-analyses demonstrated modest differences in median costs for care in the first post-transplant year, with alemtuzumab therapy ~$250 cheaper than therapies for matched patients receiving basiliximab or thymoglobulin ($228.60 and $261.70 median differences respectively; p < 0.001). Overall, the modest differences in outcomes across groups suggest, in some cases, factors like convenience and ability to tolerate side effects may be reasonable ways to select induction therapy. 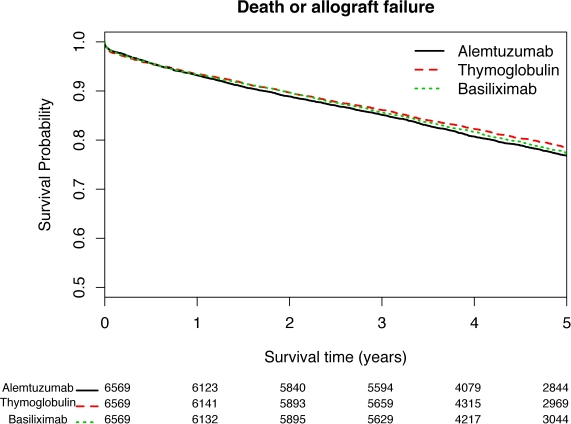
CITATION INFORMATION: Koyawala N, Silber J, Rosenbaum P, Bloom R, Wang W, Hill A, Nazarian S, Sawinski D, Trofe J, Lim M, Schold J, Reese P. Kidney Transplant Induction Therapies: Risks of Death, Allograft Failure, Sepsis, and Cancer. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Koyawala N, Silber J, Rosenbaum P, Bloom R, Wang W, Hill A, Nazarian S, Sawinski D, Trofe J, Lim M, Schold J, Reese P. Kidney Transplant Induction Therapies: Risks of Death, Allograft Failure, Sepsis, and Cancer. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/kidney-transplant-induction-therapies-risks-of-death-allograft-failure-sepsis-and-cancer/. Accessed March 5, 2026.« Back to 2016 American Transplant Congress
