Is a Single Dose of Steroids in Adult Living Donor Liver Transplant Safe? – A Cohort Analysis
University of California San Francisco, San Francisco, CA
Meeting: 2022 American Transplant Congress
Abstract number: 1086
Keywords: Immunosuppression, Post-transplant diabetes, Rejection
Topic: Clinical Science » Liver » 54 - Liver: Immunosuppression and Rejection
Session Information
Session Name: Liver: Immunosuppression and Rejection
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Steroids increase morbidity, and steroid avoidance (SA) has been studied in deceased donor liver transplant but, little is known about SA in living donor liver transplant (LDLT). We report the characteristics, and outcomes, including incidence of rejection and complications of steroid use in cohorts of LDLT patients.
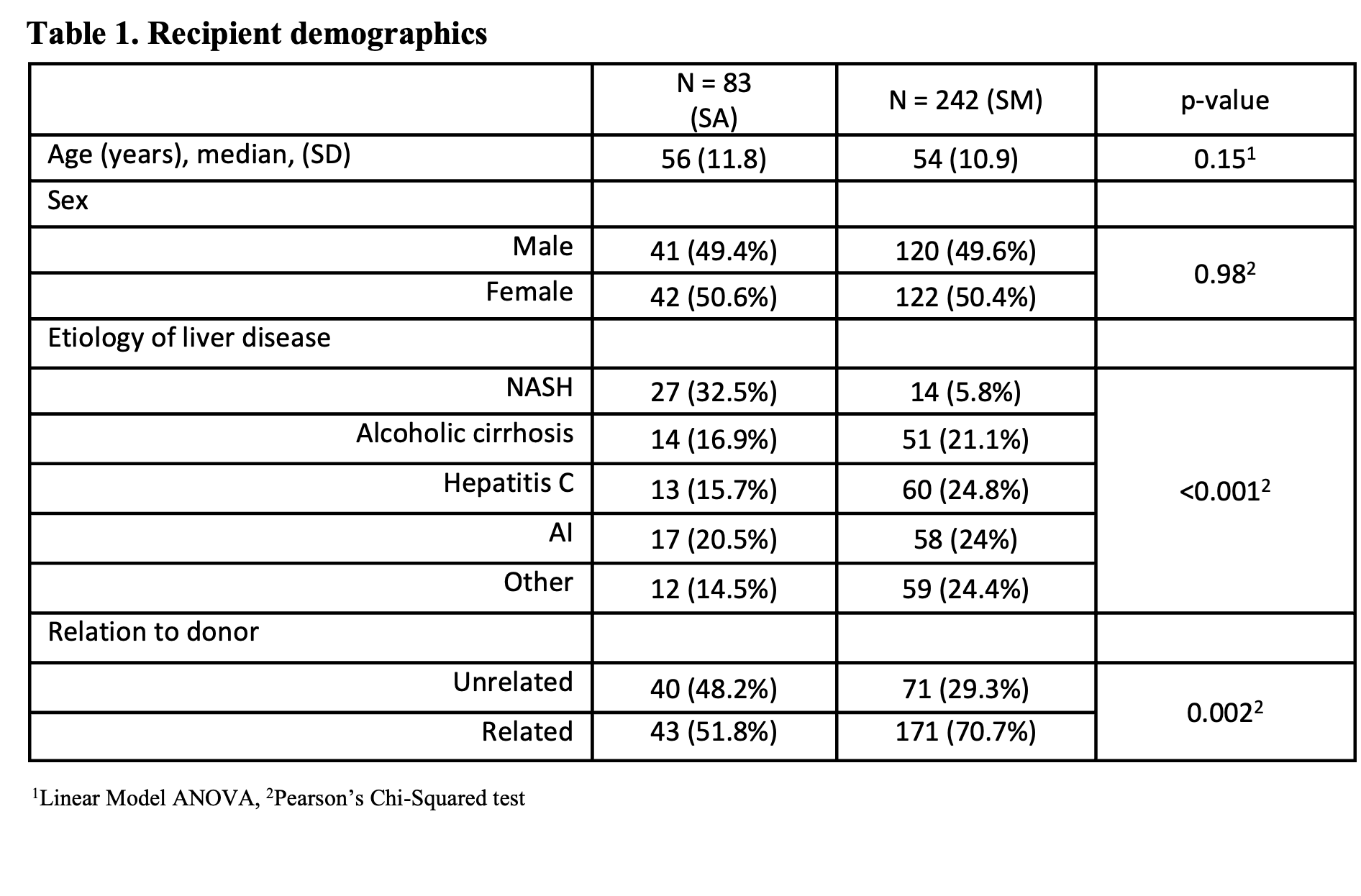
*Methods: Routine steroid maintenance (SM) after LDLT was stopped in 12/2017. Our single-center retrospective cohort includes 242 adult pts who underwent LDLT with SM (1/2000-12/2017) and 83 pts (12/2017-8/2021) with SA who received a single dose of steroids during LDLT. Acute rejection was defined as a biopsy showing pathologic characteristics within 6 months of LDLT. A univariate logistic regression was used to determine the effect of donor and recipient characteristics on rejection. Pearson’s Chi-squared test and linear model ANOVA p-values are reported.
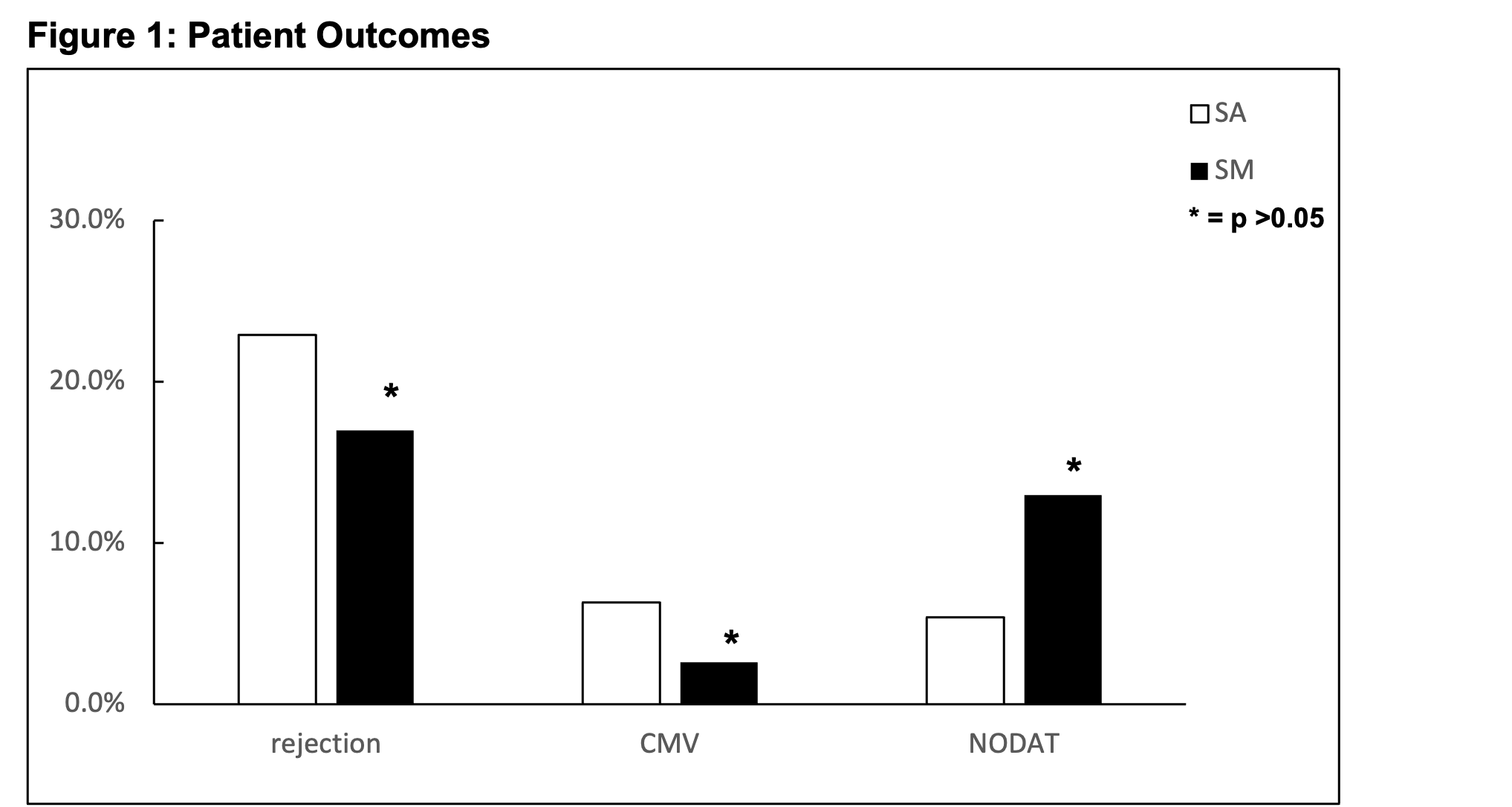
*Results: Acute rejection was not significantly different between cohorts (19/83 (22.9%) SA and 41/242 (17%) on SM, p=0.23). Subset analysis of patients with autoimmune (AI) disease also did not show a significant difference in SA (5/17 (29.4%) vs 19/58 (22.4%) on SM, p = 0.79). Using a univariate logistic regression, demographic variables including relationship of recipient to donor had no impact on rejection (OR = 0.57, 95% CI 0.32-1.01). 5 pts (6.3%) in the SA group vs 6 (2.6%) on SM developed cytomegalovirus infection (CMV) (p = 0.12). Of the patients that were not diabetic prior to LDLT, 3/56 (5.4%) with SA vs 26/200 (13%) on SM developed new-onset diabetes after transplant (NODAT) (p = 0.11).
*Conclusions: Our results demonstrate that LDLT patients on SA protocols do not exhibit higher rates of rejection than patients who received SM. Although not significant, SA had a trend towards decreased NODAT. Notably, this practice seems to be safe in pts with AI disease.
To cite this abstract in AMA style:
Nunez M, Praglin C, Torres AM, Agudelo E, Braun H, Huang C, Syed S, Roberts J, Roll G. Is a Single Dose of Steroids in Adult Living Donor Liver Transplant Safe? – A Cohort Analysis [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/is-a-single-dose-of-steroids-in-adult-living-donor-liver-transplant-safe-a-cohort-analysis/. Accessed February 19, 2026.« Back to 2022 American Transplant Congress