Interim Analysis of a Double-Blinded Placebo Controlled Trial of IVIG v. IVIG + Rituximab for Desensitization of Highly-HLA Sensitized (PRA >80%) (HS) Deceased Donor (DD) Candidates
Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
Transplant Immunology Laboratory, Cedars-Sinai Medical Cente
Meeting: 2013 American Transplant Congress
Abstract number: 153
INTRODUCTION: Desensitization protocols (DES) for HS patients are primarily aimed at living-donor recipients. The efficacy of various DES is not established, especially for DD recipients. These include plasma exchange (PLEX) + low dose IVIG, high dose (HD)-IVIG or HD-IVIG + rituximab (anti-CD20). Here, we explored the efficacy and outcomes of two DES for HS DD candidates. METHODS: From 2/11-6/12, candidates were randomized to IVIG + Placebo v. IVIG + rituximab (IVIG 2gm/kg i.v. weeks 1 & 4; rituximab 1gm or placebo week 2). Total enrollment was planned for 90 patients. End points included rates of transplantation, ABMR, SCr, protocol biopsies at 1 year, patient & graft survival and DSA assessments. RESULTS: Briefly, 15 HS-DDs were randomized and 11(73%) transplanted. However, we discontinued study entry after 4 SAEs were observed (3, ABMR and 2 graft losses). By protocol, the study was unblinded and the attribution of patients to the two protocol arms was noted (IVIG N=5, IVIG + rituximab N=6) (Table 1). No significant differences between treatment groups were seen on PRA or CMXs. All ABMR episodes occurred in the IVIG + Placebo arm and required intense therapy including PLEX + rituximab and eculizumab (anti-C5) (p=0.02). The 2 graft losses were in the placebo group (1ABMR, 1BKAN). Both IVIG and IVIG + rituximab reduced DSA levels to a range permissible for transplantation, but assessment over 6Ms showed significant rebound in the IVIG group which was associated with severe ABMR in 3/5 (60%) patients. 3/6 patients in the IVIG + rituximab arm had protocol biopsies, none with ABMR.
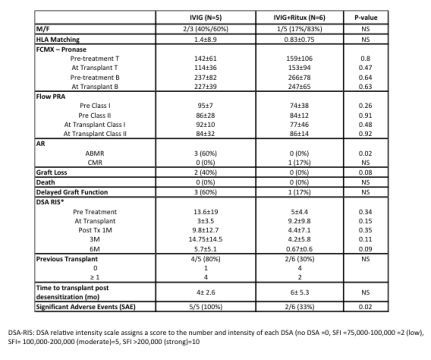
CONCLUSIONS: Based on limited assessment of this placebo controlled trial, both IVIG and IVIG + rituximab are effective in reducing DSA and CMXs to a level allowing transplantation of HS DD recipients. However, IVIG + rituximab was more effective in preventing DSA rebound, ABMR episodes & graft losses. NCT01178216.
Jordan, S.: Grant/Research Support, Genentech, Inc. CSL Behring.
To cite this abstract in AMA style:
Vo A, Choi J, Lukovsky M, Toyoda M, Ge S, Kahwaji J, Peng A, Villicana R, Najjar R, Jordan S. Interim Analysis of a Double-Blinded Placebo Controlled Trial of IVIG v. IVIG + Rituximab for Desensitization of Highly-HLA Sensitized (PRA >80%) (HS) Deceased Donor (DD) Candidates [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/interim-analysis-of-a-double-blinded-placebo-controlled-trial-of-ivig-v-ivig-rituximab-for-desensitization-of-highly-hla-sensitized-pra-80-hs-deceased-donor-dd-candidates/. Accessed February 28, 2026.« Back to 2013 American Transplant Congress
