Infectious Complications Associated with Eculizumab Used for ABO-Incompatible Renal Transplantation
University of Illinois at Chicago, Chicago.
Meeting: 2018 American Transplant Congress
Abstract number: D165
Keywords: Infection, Kidney transplantation, Outcome
Session Information
Session Name: Poster Session D: Kidney Infectious
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Introduction: A novel approach in ABO-incompatible (ABOi) living donor renal transplant (LDRT) includes use of eculizumab (ECU) that allows for avoidance of traditional desensitization. We report infectious outcomes in a series of 4 cases that underwent ABOi LDRT using ECU.
Case Series: Four patients received ECU 1200mg IV on POD -1, followed by 900mg on POD1, 7, 14, 21, 28 and 1200mg week 5, 7 and 9. Patients received TPEX until ECU administration. Patients did not receive TPEX, IVIG or splenectomy post-LDRT. Patients 1 and 4 received LDRT despite ABO titer over 1:4 – a traditional cut off. Rabbit ATG induction was used with TAC, MPA and a rapid steroid taper. Patients 1, 3 and 4 had an uncomplicated post-op course. Patient 1 had protocol biopsies at month 3 and 6 showing C4d positivity in the setting of negative DSA and elevated ABO titer. Patient 2 had a complicated post-op course with peri-operative NSTEMI leading to DGF requiring HD from POD 4-45. Biopsy on POD28 showed ATN with mild focal C4d positivity (total score 1), DSA was negative and ABO titer was 1:8. Subsequently, Patient 2 was started on prednisone on POD35 due to concern for rejection in the setting of subtherapeutic TAC levels and DGF.
All patients received meningococcal, pneumococcal and haemophilus vaccines before starting TPEX, with penicillin VK 500 mg BID administered until 4 weeks after last ECU dose. Patients received standard prophylaxis with Bactrim and valganciclovir 450 mg daily for 6 months. No meningococcal infections occurred to date. Patient 1 had a urinary tract infection on POD30 treated successfully with nitrofurantoin. Patient 2 developed Clostridium difficile infection on POD30 treated with 6-week course of oral vancomycin. She also developed resistant cytomegalovirus (CMV) viremia at 5 months that resolved after 7 months of high dose valganciclovir and MPA discontinuation (D+/R-; peak CMV PCR 170,000 copies). No other patients experienced CMV, EBV or BK infections.
Discussion: ECU use for ABOi LDRT resulted in excellent outcomes and did not result in serious infectious complications in our case series.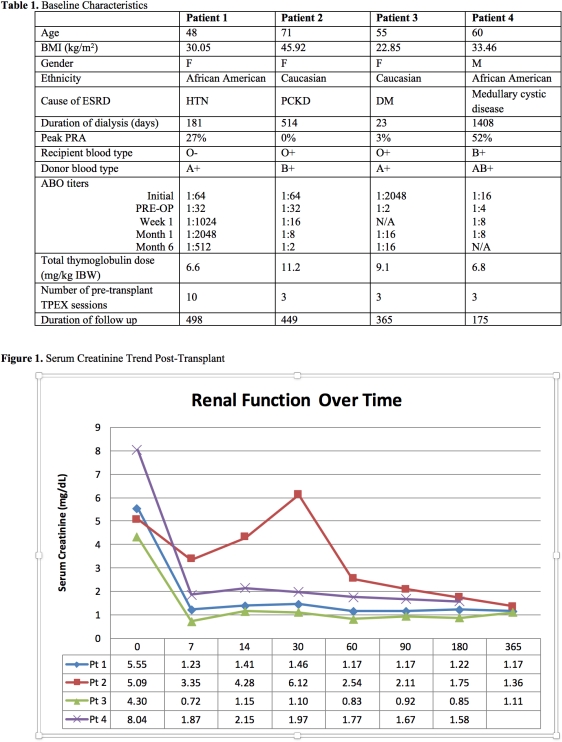
CITATION INFORMATION: Campara M., West-Thielke P., Progar K., Jasiak N., Lichvar A., Tang I., Benedetti E. Infectious Complications Associated with Eculizumab Used for ABO-Incompatible Renal Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Campara M, West-Thielke P, Progar K, Jasiak N, Lichvar A, Tang I, Benedetti E. Infectious Complications Associated with Eculizumab Used for ABO-Incompatible Renal Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/infectious-complications-associated-with-eculizumab-used-for-abo-incompatible-renal-transplantation/. Accessed February 23, 2026.« Back to 2018 American Transplant Congress
