In Depth Look at Acute Rejections in LCP-Tacrolimus (LCPT; Envarsus XR) and Immediate-Release Tacrolimus (IR-Tac)-Treated De Novo Kidney Transplant Recipients
1University of New Mexico Hospitals, Albuquerque, NM, 2University of Texas Medical Branch, Galveston, TX, 3Veloxis Pharmaceuticals Inc., Cary, NC
Meeting: 2019 American Transplant Congress
Abstract number: C158
Keywords: FK506, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session C: Kidney: Acute Cellular Rejection
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: A prospective, randomized controlled trial has shown comparable rates of biopsy-proven rejection (BPAR) between LCPT and IR-Tac treated renal transplant recipients. This analysis aims to examine in more detail the BPAR severity, kidney function, and outcomes in those with BPAR.
*Methods: A post-hoc analysis of 536 kidney transplant recipients from a large Phase 3 study of LCPT vs. IR-Tac in de novo kidney transplant recipients was conducted. Biopsy reports evaluated by central pathologists were reviewed to determine rejection severity. “Rejection creatinine” was defined as the last creatinine immediately prior to the date of biopsy, and “nadir creatinine” was the average of the 3 lowest creatinine concentrations within 6 months of the BPAR.
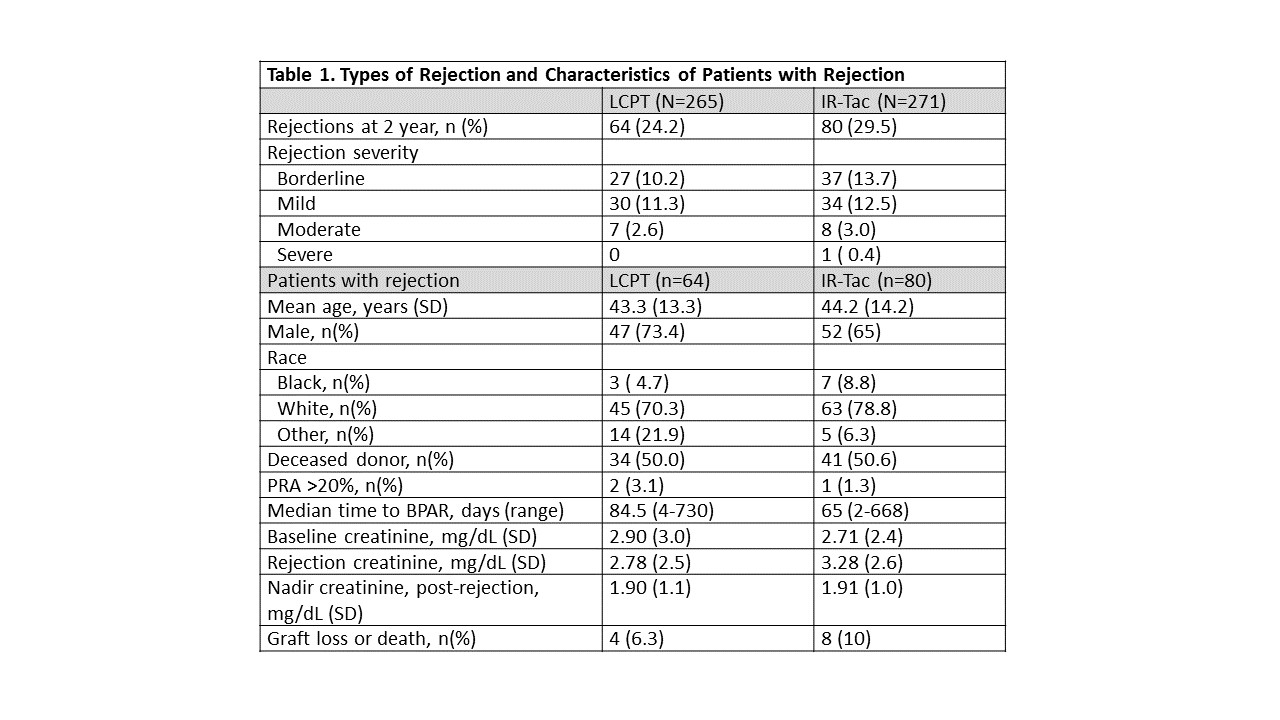
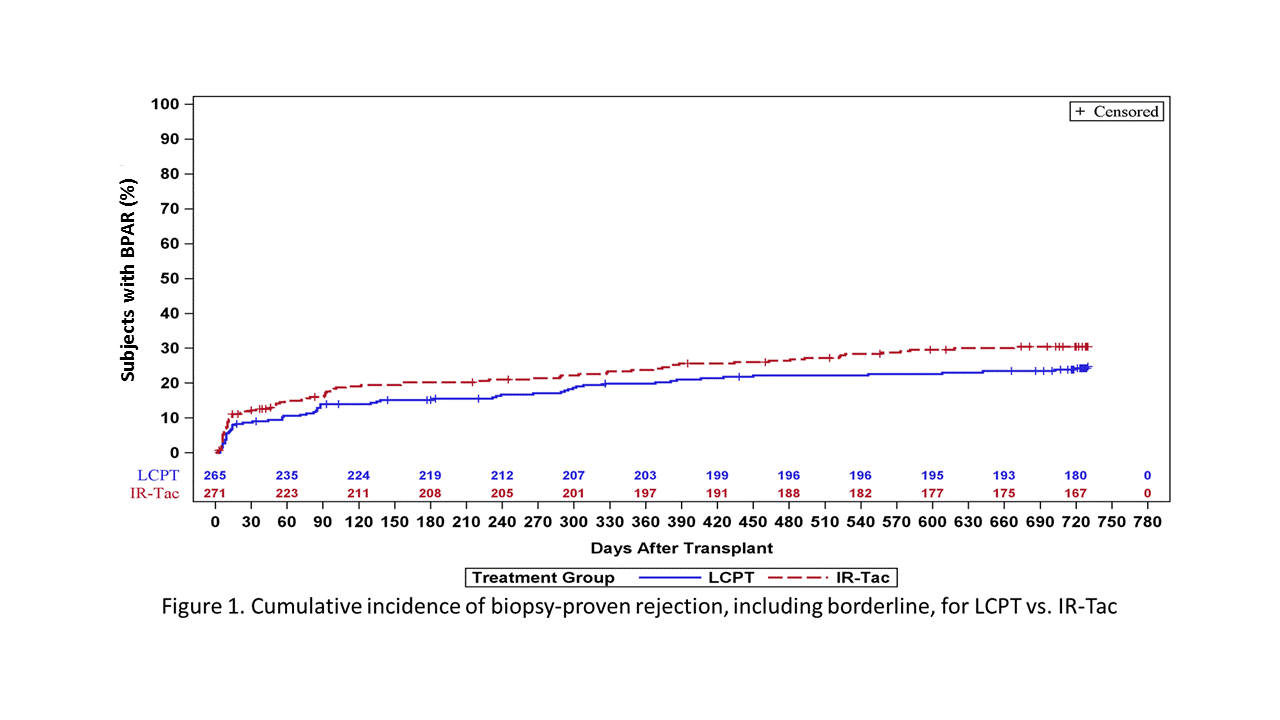
*Results: Demographic and rejection characteristics of the 64 LCPT (24.2%) and 80 IR-Tac (29.5%) patients who experienced BPAR are shown in Table 1. Severity of BPAR episodes were comparable between the groups. Similarly, renal function during and post BPAR was similar for both groups. Kaplan-Meier analysis of the incidence of BPAR is shown in Figure 1.
*Conclusions: These data indicate that incidence of rejection and rejection characteristics between LCPT and IR-Tac are similar with regard to severity, impact on renal function, and impact on overall outcomes.
To cite this abstract in AMA style:
Condon A, Mujtaba M, Patel SJ, Stevens DR, Meier-Kriesche U. In Depth Look at Acute Rejections in LCP-Tacrolimus (LCPT; Envarsus XR) and Immediate-Release Tacrolimus (IR-Tac)-Treated De Novo Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/in-depth-look-at-acute-rejections-in-lcp-tacrolimus-lcpt-envarsus-xr-and-immediate-release-tacrolimus-ir-tac-treated-de-novo-kidney-transplant-recipients/. Accessed February 26, 2026.« Back to 2019 American Transplant Congress