Improved Bioavailability and Reduced Dose Requirements of Novel, Once-Daily, MeltDose® Tacrolimus Formulation Tablets (Envarsus® XR) Compared to Twice-Daily Tacrolimus Capsules (Prograf®) in De Novo Kidney Transplantation: Results of a Phase 3 Double-Dummy, Randomized Trial
1University of Barcelona, Catalunya, Barcelona, Spain
2David Geffen School of Medicine at UCLA, Los Angeles
3St. Barnabas Medical Center, Livingston
4California Institute of Renal Research, San Diego.
Meeting: 2015 American Transplant Congress
Abstract number: B67
Keywords: Immunosuppression
Session Information
Session Name: Poster Session B: Clinical Science: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Sunday, May 3, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Tacrolimus (tac) is an immunosuppressive drug with a narrow therapeutic range. Adequate trough levels following kidney transplantation (≥6 ng/mL depending on regimen and time post-transplant) are required to prevent against rejection while Cmax cannot be too high (generally <12ng/mL) or else there is risk for toxicity and adverse events. Contributing to tac dosing challenges are suboptimal pharmacokinetics (PK; e.g., low bioavailability, high fluctuation) of the traditional twice-daily capsule formulation (Prograf®) and differences in tac metabolism among specific patient populations (e.g., African Americans; females). Envarsus® XR is an extended-release, once-daily, formulation of tac that uses a novel MeltDose® drug delivery technology which decreases drug particle size down to a molecular level resulting in improved absorption and once-daily dosing. Randomized trials in de novo and stable kidney transplant recipients have shown increased bioavailability, reduced Cmax and noninferior efficacy and similar safety as Prograf, at a reduced total daily dose (TDD). The purpose of the present analysis was to examine dosing and bioavailability (trough/TDD) of tac over a two year period in de novo kidney recipients randomized to Envarsus XR or Prograf. Immediately post-transplant, target tac trough levels were more rapidly achieved in the Envarsus XR group. From Week 3 onward, TDDs were lower for Envarsus XR and the difference between the two groups increased over time. By month 24, the mean TDD for Envarsus XR was 24% lower than the Prograf group, yet troughs (ng/mL) were similar (5.5 vs. 5.7, respectively). These data show that Envarsus XR is associated with continued improved absorption/bioavailability, resulting in the requirement for a lower TDD vs. Prograf, over two years.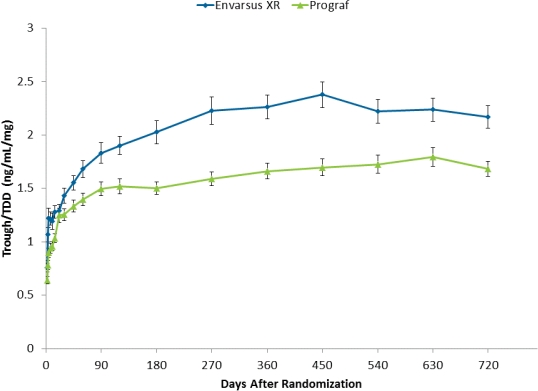
To cite this abstract in AMA style:
Grinyó J, Bunnapradist S, Mulgaonkar S, Steinberg S. Improved Bioavailability and Reduced Dose Requirements of Novel, Once-Daily, MeltDose® Tacrolimus Formulation Tablets (Envarsus® XR) Compared to Twice-Daily Tacrolimus Capsules (Prograf®) in De Novo Kidney Transplantation: Results of a Phase 3 Double-Dummy, Randomized Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/improved-bioavailability-and-reduced-dose-requirements-of-novel-once-daily-meltdose-tacrolimus-formulation-tablets-envarsus-xr-compared-to-twice-daily-tacrolimus-capsules-prograf/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
