Implementing Mobile Health Technology for Living Kidney Donor Follow-Up: Transplant Provider Perspectives
1Department of Surgery, Johns Hopkins School of Medicine, Baltimore
2Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: A382
Session Information
Session Name: Poster Session A: Quality Assurance Process Improvement
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Transplant hospitals are required to collect and report living kidney donor (LKD) follow-up data at 6-months, 1-year, and 2-years, but fewer than 50% of hospitals currently meet required thresholds. Mobile health (mHealth) technologies could ease documented logistical burdens in collecting these data, but these technologies have not yet been explored or implemented.
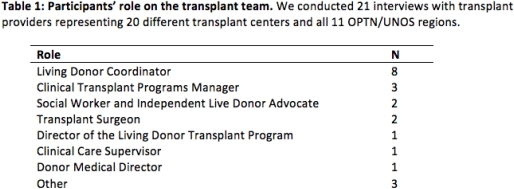
METHODS: We conducted semi-structured interviews with 21 transplant providers representing all 11 OPTN/UNOS regions. Interviews included questions about current processes for counseling LKDs about follow-up care, and insight into implementation strategies for a novel mHealth system to capture LKD follow-up data. We transcribed and analyzed interviews for thematic analysis using NVivo Pro 11.
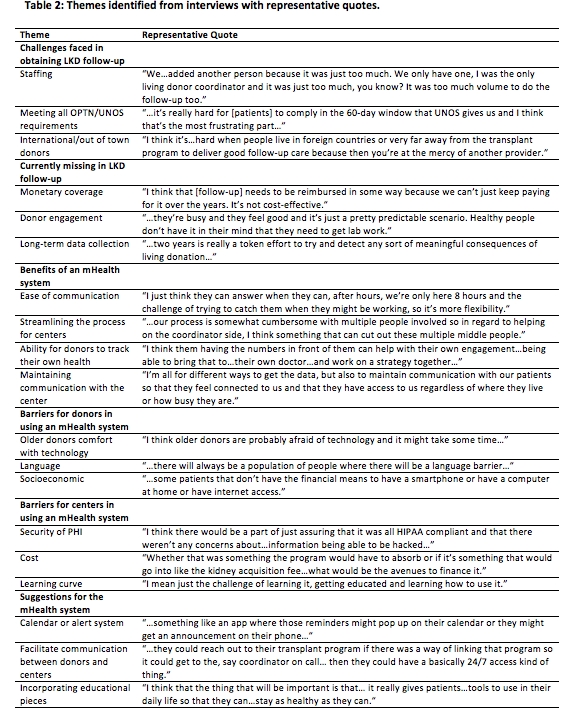
RESULTS: Transplant hospitals currently face challenges with donor engagement, international/out of town donors, and adequate staffing to fulfill OPTN/UNOS follow-up mandates. They believe 2 years is not long enough to follow LKDs, but struggle to keep donors engaged for even the 2-year time period, and lack the resources to continue to follow these donors. While there is general optimism for the use of mHealth technology, some concerns include language and socioeconomic barriers, cost, security of protected health information, older donors' comfort with the technology, and the hospital's ability to learn and adopt the technology.
CONCLUSIONS: mHealth has the potential to ease transplant hospital burden for collecting and reporting OPTN/UNOS-required LKD follow-up data, and an mHealth system designed for this purpose should meet national standards for protecting health information, engage providers in implementation, and be low cost to adopt nationally.
CITATION INFORMATION: Eno A., Ruck J., Rasmussen S., Segev D., Henderson M. Implementing Mobile Health Technology for Living Kidney Donor Follow-Up: Transplant Provider Perspectives Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Eno A, Ruck J, Rasmussen S, Segev D, Henderson M. Implementing Mobile Health Technology for Living Kidney Donor Follow-Up: Transplant Provider Perspectives [abstract]. https://atcmeetingabstracts.com/abstract/implementing-mobile-health-technology-for-living-kidney-donor-follow-up-transplant-provider-perspectives/. Accessed March 1, 2026.« Back to 2018 American Transplant Congress