Impact of the Covid-19 Pandemic on Longitudinal Molecular Surveillance in the KOAR Registry
T. Alhamad1, A. Malone1, D. Mandelbrot2, P. M. Anand3, H. M. Wadei4, G. Shekhtman5, N. Agrawal5, K. Pinney5, K. Qu5, A. Desai6
1Washington University, St. Louis, MO, 2University of Wisconsin, Madison, WI, 3Medical University of South Carolina, Charleston, SC, 4Mayo Clinic Jacksonville, Jacksonville, FL, 5CareDx, Brisbane, CA, 6Loyola University Medical Center, Maywood, IL
Meeting: 2022 American Transplant Congress
Abstract number: 1606
Keywords: COVID-19, Kidney transplantation, Monitoring, Rejection
Topic: Clinical Science » Ethics » 22 - Psychosocial and Treatment Adherence
Session Information
Session Name: Psychosocial and Treatment Adherence
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The SARS-CoV2 pandemic increased the complexity of delivering routine clinical care and laboratory services for immunosuppressed kidney transplant (KTx) recipients. We evaluated how the pandemic impacted adherence with scheduled laboratory draws among patients enrolled in the Kidney allograft Outcomes AlloSure Registry (KOAR, NCT03326076).
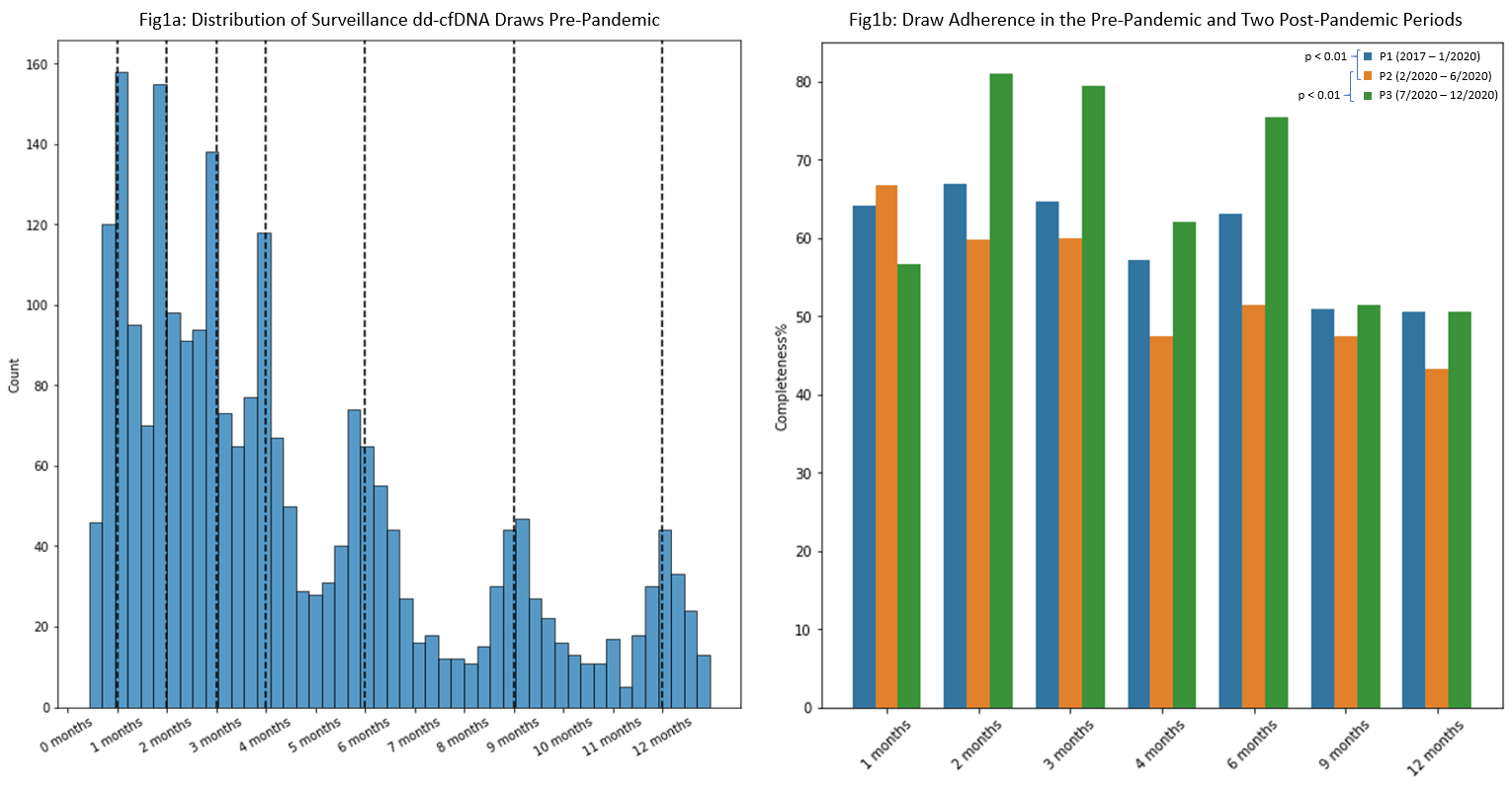
*Methods: 1663 kidney transplant (KTx) recipients undergoing post-transplant surveillance using donor-derived cell-free DNA (dd-cfDNA, AlloSure®, CareDx Inc.) were enrolled in KOAR between 2017 and 2021. Participating centers were free to individualize their surveillance strategies. We estimated adherence by using the pre-pandemic distribution of surveillance dd-cfDNA draws across participating sites to establish a baseline regimen, and then compared adherence before the pandemic (P1; through 1/2020) with two subsequent periods in 2020: P2 (2/2020 – 6/2020), coinciding with the first wave of infections, and P3 (7/2020 – 12/2020), which captures the bulk of the second and third waves in the US.
*Results: The distribution of surveillance dd-cfDNA draws at participating sites before COVID (P1) identified 7 peaks corresponding to draw points at or around months 1, 2, 3, 4, 6, 9, and 12 [Figure 1a]. Estimated adherence during P1 based on this regimen was 60.5%. Over the subsequent 5 months (P2), reflecting the early months of the pandemic, adherence declined to 50.5% (p < 0.01). After the expanded availability of mobile phlebotomy services in 7/2020 and despite rising SARS-CoV2 case counts and hospitalizations, adherence during P3 improved to 57.6% (p < 0.01 compared to P2, p = 0.1 compared to P1) [Figure 1b].
*Conclusions: Our findings demonstrate that adherence to laboratory surveillance among transplant recipients enrolled in the KOAR registry declined in the early period of the SARS-CoV2 pandemic, however, a variety of adaptations in the latter half of 2020, including the widespread availability of remote phlebotomy for these patients, appears to have led to substantial improvements, with adherence approaching pre-pandemic levels.
To cite this abstract in AMA style:
Alhamad T, Malone A, Mandelbrot D, Anand PM, Wadei HM, Shekhtman G, Agrawal N, Pinney K, Qu K, Desai A. Impact of the Covid-19 Pandemic on Longitudinal Molecular Surveillance in the KOAR Registry [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-the-covid-19-pandemic-on-longitudinal-molecular-surveillance-in-the-koar-registry/. Accessed March 1, 2026.« Back to 2022 American Transplant Congress