Impact of Repeat Testing of Living Organ Donors after Implementation of a Protocol To Repeat Testing within 14 Days of the Transplant Procedure: A Multi-Center Retrospective Survey
Division of Infectious Diseases and Organ Transplantation, Northwestern University, Chicago
Division of Nephrology, Columbia University College of Physicians and Surgeons, New York
Recanati Miller Transplant Institute, Mt. Sinai Hospital, New York
Meeting: 2013 American Transplant Congress
Abstract number: A799
Background: Transmission of HIV by a live donor prompted the NY State Dept of Health and CDC to recommend that all live donors undergo additional screening for HIV, HBV and HCV within 7-14 days of the donation procedure in March 2011. There is controversy about this recommendation, including concerns that such screening will result in delays and cancellations of transplantation procedures.
Methods: We surveyed all live donor transplant centers in New York State via an anonymous SurveyMonkey.com survey to assess how they have implemented protocols to screen live organ donors within 14 days of the transplant procedure and to assess the outcomes of the screening protocols.
Results: Eight of 11 surveyed NY State live donor programs (8 kidney, 4 liver, 1 lung) responded to the survey (Damage from Hurricane Sandy delayed response from 1 center). All but 1 program has a formal policy to ensure repeat donor screening within 14 days of procedure. The screening program has not resulted in any cancellations of cases, but 2 centers experienced delays in transplantation due to the retesting policy generally as the result of technician and laboratory procedural mistakes which necessitated repeat phlebotomy. Testing is typically accomplished during pre-surgical visit, when other labs and H&Ps are performed (see table 1 for screening protocols). Serology was most frequently used in the initial evaluation, whereas repeat testing included NAT (100%) and serology (37-62%) (Table 2).
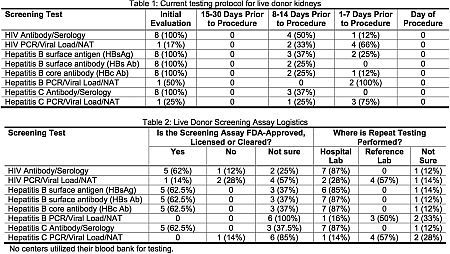
Conclusions: Most (88%) responding centers have policies to retest living donors with 14 days of the transplant procedures as recommended by the NY State Dept of Health. Such policies have resulted in rare delays of scheduled transplants in few centers and no cancelations.
To cite this abstract in AMA style:
Echenique I, Cohen D, Rudow DLaPointe, Ison M. Impact of Repeat Testing of Living Organ Donors after Implementation of a Protocol To Repeat Testing within 14 Days of the Transplant Procedure: A Multi-Center Retrospective Survey [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/impact-of-repeat-testing-of-living-organ-donors-after-implementation-of-a-protocol-to-repeat-testing-within-14-days-of-the-transplant-procedure-a-multi-center-retrospective-survey/. Accessed February 28, 2026.« Back to 2013 American Transplant Congress
