Hepatitis C Genotypes Among Deceased Organ Donors in the United States.
1University of Pennsylvania, Philadelphia, PA
2Gift of Life, Philadelphia, PA
Meeting: 2017 American Transplant Congress
Abstract number: C82
Keywords: Allocation, Donation, Hepatitis C
Session Information
Session Name: Poster Session C: Donor Management: All Organs
Session Type: Poster Session
Date: Monday, May 1, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Background: HCV is one of the leading causes of liver disease in the US. 5% of deceased organ donors are HCV+ and HCV infection is commonly first detected through the donor screening process. Although nucleic acid testing (NAT) is performed on nearly all deceased-donors in the US, HCV genotyping is not. Thus the HCV genotype distribution of deceased-donors is unknown, which might affect HCV treatment post-transplant.
Methods: HCV NAT+ deceased donors who met eligibility criteria as kidney donors to HCV-negative recipients in the THINKER (Transplanting Hepatitis C Kidneys Into Negative KidnEy Recipients;NCT02743897) study had HCV genotype testing performed with a laboratory developed procedure using the eSensor® HCVg Direct Test (RUO) and XT-8 System (GenMark Diagnostics, Carlsbad, CA). Genotyping was performed in real-time during organ allocation when any THINKER candidate had priority on the match run to plausibly receive the kidney.
Results: Between 6/1/16-11/10/16, 28 HCV NAT-positive deceased donors were genotyped. Of these 28 donors, 20 (71.4%) were from region UNOS region 2, with the remainder from UNOS regions 1, 3, 7, 10, and 11. Active intravenous drug use was noted in 27 (96.4%) of these donors (one had prior use), and 24 (85.7%) died from a drug overdose. The genotype distribution (Figure 1), and is significantly different from the genotype distribution in the US based on 2010 NHANES data and data from Kaiser Permanente Northern California.
Conclusions: Compared to the broader HCV population, this sample suggests that HCV+ deceased donors, the majority of whom are active IV drug users, are less likely to be genotype 1 and more likely genotype 3. This finding has important implications, including: a) the potential for mixed HCV infections in the recipient; b) inability to treat certain genotypes (i.e., 2 and 3) with Sofosbuvir-based regimens if post-transplant kidney function is poor; and challenges with post-transplant insurance authorization if pan-genotypic regimens are not part of an insurer's formulary.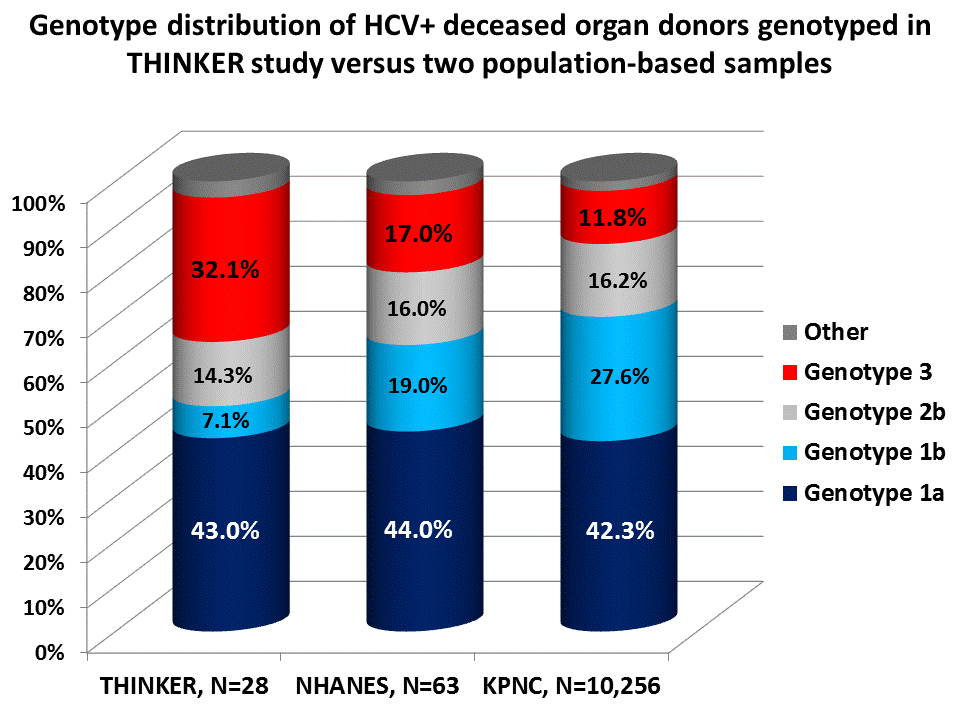
CITATION INFORMATION: Goldberg D, Van Deerlin V, Farooqi M, Sicilia A, Hasz R, Bloom R, Blumberg E, Gentile C, Abt P, Reese P. Hepatitis C Genotypes Among Deceased Organ Donors in the United States. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Goldberg D, Deerlin VVan, Farooqi M, Sicilia A, Hasz R, Bloom R, Blumberg E, Gentile C, Abt P, Reese P. Hepatitis C Genotypes Among Deceased Organ Donors in the United States. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/hepatitis-c-genotypes-among-deceased-organ-donors-in-the-united-states/. Accessed March 1, 2026.« Back to 2017 American Transplant Congress
