HCV, Acute Rejection, and the New Direct-Acting Antivirals in Deceased Donor Kidney Transplant Recipients.
JHU, Baltimore.
Meeting: 2016 American Transplant Congress
Abstract number: D3
Keywords: Hepatitis C, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session D: Antibody Mediated Rejection: Session #2
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
The association between hepatitis C virus (HCV) seropositivity and acute rejection (AR) in kidney transplant donors and recipients is unclear, and may have changed with the advent of the new direct-acting antiviral (DAA) treatments for HCV.
METHODS: Using SRTR data, we studied donor and recipient HCV and AR in 91,556 deceased donor kidney-only transplantations (DDKT) from 1/2004 to 3/2015. We used multilevel logistic regression (adjusting for donor and recipient characteristics and accounting for center-level variation) to assess the association between HCV serostatus and AR, with interaction terms to test whether this association varied by pre-/post-DAA eras (before versus after 12/6/2013).
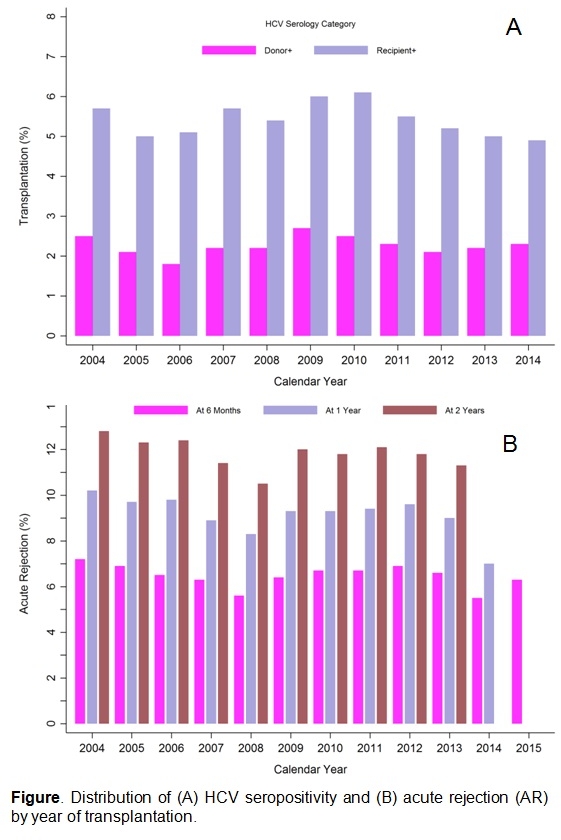
RESULTS: 5.4% of DDKT recipients were HCV+; 2.3% received a kidney from an HCV+ donor (Figure A). Among HCV+ recipients, 38.9% received HCV+ donor kidneys. Among recipients of HCV+ and HCV- donor kidneys, 19.2% and 14.2% experienced AR during follow-up, respectively. Among HCV+ and HCV- recipients, 17.4% and 14.0% experienced AR, respectively. Most AR episodes were diagnosed during the first year (Figure B). Receiving an HCV+ donor kidney was independently associated with higher risk of AR within the first year (aOR:1.34, p=0.007), while recipient HCV was independently associated higher risk of AR beyond the first year (aOR1.30, p=0.002) (Table). There was no evidence of attenuation in the association between donor (p=0.9) or recipient (p=0.8) HCV and AR in the post-DAA era.
|
Time at AR Diagnosis |
aOR for HCV |
Intraclass Correlation Coefficient (%) |
|||
|
Recipient+ |
P |
Donor+ |
P |
||
|
Within First Year |
0.941.091.26 |
0.2 |
1.081.341.65 |
0.007 |
11.9 |
|
Beyond First Year |
1.101.301.54 |
0.002 |
0.650.861.13 |
0.3 |
5.9 |
CONCLUSIONS: HCV seropositivity in DDKT donor and recipient was associated with higher risk of AR. Immunosuppression and antiviral treatments need to be optimized for transplantations involving HCV+ donors or recipients. The effectiveness of DAA in improving HCV+ kidney transplantation outcomes awaits further evaluation when adequate data on long-term follow-up become available.
CITATION INFORMATION: Liao C, Massie A, Bae S, Segev D. HCV, Acute Rejection, and the New Direct-Acting Antivirals in Deceased Donor Kidney Transplant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Liao C, Massie A, Bae S, Segev D. HCV, Acute Rejection, and the New Direct-Acting Antivirals in Deceased Donor Kidney Transplant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/hcv-acute-rejection-and-the-new-direct-acting-antivirals-in-deceased-donor-kidney-transplant-recipients/. Accessed February 21, 2026.« Back to 2016 American Transplant Congress
