HCV Ab+/NAT- Organ Donors, and the Challenges of 'Eclipse Windows': Analysis of the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee (DTAC)
DTAC, United Network for Organ Sharing, Richmond, VA.
Meeting: 2018 American Transplant Congress
Abstract number: 569
Keywords: Donation, Hepatitis C, Infection
Session Information
Session Name: Concurrent Session: New Frontiers in HIV and Hepatitis
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Room 4C-3
Introduction: In 2017, CDC reported new hepatitis C (HCV) cases had tripled over 5 years, mainly due to intravenous drug use (IVDU). As the proportion of deceased donors with a history of IVDU continues to increase, we report the DTAC experience with HCV cases.
Methods: The multidisciplinary DTAC evaluates and adjudicates potential donor-derived transmission events. 15 reports of HCV were reviewed, from 1/1/17 through 10/31/17. 6 were from seropositive (Ab+/NAT-) donors and 9 from Ab-/NAT- donors. Historical DTAC cases adjudicated as either proven or probable (p/p) eclipse NAT-window infections (7) were also reviewed. Cases without donor transmission were deemed excluded.
Results: Amongst Ab+/NAT- donor cases, 5/6 were p/p, 1 excluded. Only livers were infected, 6 other organs were excluded (kidneys, lungs, heart). Amongst HCV Ab-/NAT– donors, 2 donors resulted in p/p transmissions, 7 were excluded. All 7 p/p donors came from different OPOs, in different months, and from different UNOS regions.
7 historical eclipse window donors (all Ab-/NAT- donors, 2012 – 2016) transmitted HCV to 9/14 kidneys, 3/4 hearts, 1/2 livers, and all 3 lung recipients. Combining all eclipse-window infections, all were US PHS increased risk donors, 13/14 had either active IVDU or drug use with unknown injection practices. 9/14 donors had HCV NAT drawn ≤48hrs after admission, and 5/14 within 24hrs (median = 40hrs). Time from HCV NAT testing to cross-clamp varied from 21 – 114hrs (median = 45.5hrs). 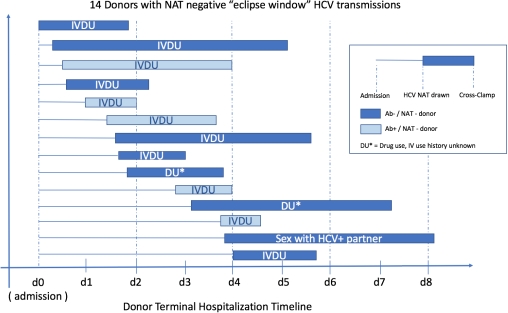
Conclusions: An increase in HCV transmission has been noted in 2017, as many in the previous 5 years combined, including for the first time from Ab+/NAT- donors in liver recipients. Eclipse NAT-window period infections remain rare, however especially in active IV drug users the timing of NAT tests in relation to admission and cross-clamp may be important.
CITATION INFORMATION: Wolfe C., Tlusty S., Vece G., Sawyer R., Bag R., Berry G., Bucio J., Danziger-Isakov L., Goldberg D., Ho C., Florescu D., Klassen D., La Hoz R., Lilly K., Mehta A., Malinis M., Nalesnik M., Theodoropoulos N., Wood P., Michaels M. HCV Ab+/NAT- Organ Donors, and the Challenges of 'Eclipse Windows': Analysis of the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee (DTAC) Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wolfe C, Tlusty S, Vece G, Sawyer R, Bag R, Berry G, Bucio J, Danziger-Isakov L, Goldberg D, Ho C, Florescu D, Klassen D, Hoz RLa, Lilly K, Mehta A, Malinis M, Nalesnik M, Theodoropoulos N, Wood P, Michaels M. HCV Ab+/NAT- Organ Donors, and the Challenges of 'Eclipse Windows': Analysis of the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee (DTAC) [abstract]. https://atcmeetingabstracts.com/abstract/hcv-ab-nat-organ-donors-and-the-challenges-of-eclipse-windows-analysis-of-the-optn-unos-ad-hoc-disease-transmission-advisory-committee-dtac/. Accessed March 11, 2026.« Back to 2018 American Transplant Congress
