Four Eyes Are Better Than Two: Improving Laboratory Report Quality Control
1Quality Assurance, LABS, Inc., Centennial, CO
2Infectious Disease, LABS, Inc., Philadelphia, PA.
Meeting: 2018 American Transplant Congress
Abstract number: A390
Keywords: Donation, Monitoring, Safety
Session Information
Session Name: Poster Session A: Quality Assurance Process Improvement
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Background: Our STAT donor-screening lab implemented a new LIMS that is used to manage patient orders, testing and reporting. We intentionally customized the LIMS to replace manual work activities, in an effort to improve quality. We are a small-sized reference laboratory, in a non-hospital based setting. We saw a reduction in requisition data entry and ABO analysis/reporting errors as a result of our LIMS enhancements.
Methods: Quality Indicator trend data was reviewed over a two year period. Two areas of improvement included (i) revised report issuance due to incorrect data entry of demographic information and (ii) ABO result interpretation and reporting. LIMS implementation occurred in October 2016 and a retrospective analysis of data was performed for the 12 months prior to and post-implementation.
Results: The new LIMS was designed to include double-blind entry of test requisition information. Two technologists independently enter data from the requisition. The LIMS flags discrepancies and allows for corrections. A 40% decrease in errors was observed due to double-blind entry (13,600 requisitions). Certain errors, such as handwriting interpretation, will remain. 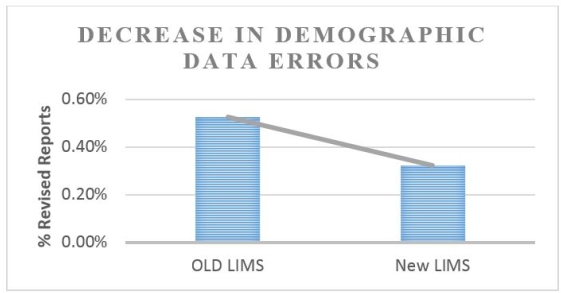
ABO accuracy is vital when allocating organs. Agglutination reactions are now entered into the LIMS. We validated the LIMS to perform the agglutination analysis and compare the results obtained by two technologists, resulting in a 100% accuracy rate for ABO interpretation. There have been no ABO result interpretation/reporting errors as a direct result of the LIMS change (5,400 tests). 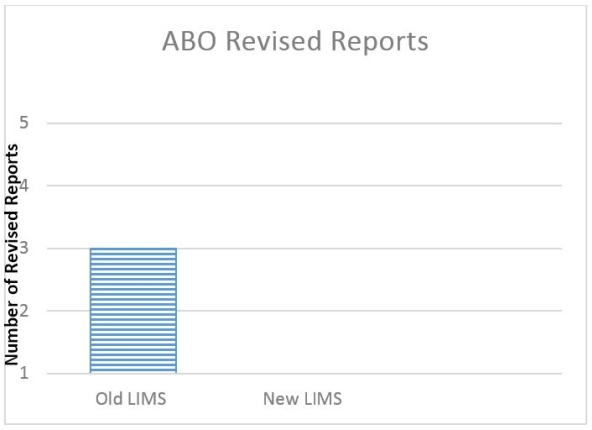
Conclusion: Leveraging our QI data, we were able to design automated processes to streamline lab functions for continuous improvement. As the data demonstrate here, we were successful in reducing report errors thereby impacting the integrity of records and patient care.
CITATION INFORMATION: Dionne S., Klama K., Rashid H., Niedzinski A. Four Eyes Are Better Than Two: Improving Laboratory Report Quality Control Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Dionne S, Klama K, Rashid H, Niedzinski A. Four Eyes Are Better Than Two: Improving Laboratory Report Quality Control [abstract]. https://atcmeetingabstracts.com/abstract/four-eyes-are-better-than-two-improving-laboratory-report-quality-control/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress
