Follow-Up Reporting for Living Kidney Donors
UNOS, Richmond, VA
U of Pittsburgh, Pittsburgh, PA
U of Iowa, Iowa City, IA
Meeting: 2013 American Transplant Congress
Abstract number: 195
Follow-up reporting for living kidney donors (LKDs) increases knowledge of short-term risks of donation. Anecdotal evidence suggests that low reporting rates are due to LKDs declining follow-up and to disadvantages of small programs (e.g., too few resources) and large programs (e.g., overwhelming volume).
The cohort was LKDs who donated 1/1/07 – 12/31/10 in the US (n=23,978). LKDs with organs not transplanted at the recovery hospital were excluded. Outcomes were timely reporting of LDKs’ lab values (serum creatinine, urine protein) and clinical data (dialysis, hypertension, diabetes, complications, readmission, working for income, and COD, if applicable) on 1-yr OPTN Living Donor Follow-up forms. Data were considered timely if patient status date was within 2 months of donation anniversary.
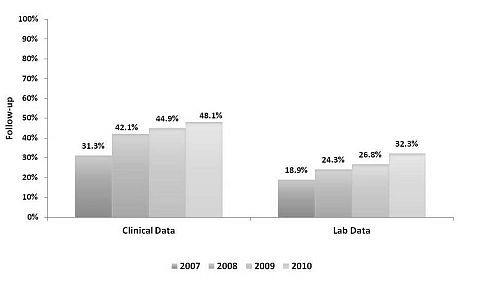
Rates of timely clinical data increased from 31% of LKDs who donated in 2007 to 48% of LKDs in 2010. 19% of LKDs in 2007 had timely lab data, vs. 32% in 2010. 16% of LKDs in 2007 had both timely clinical and lab data, vs. 27% in 2010. 19% of programs reported both lab and clinical data for at least 50% of LKDs in 2007, vs. 27% of programs for LKDs in 2010. 27% of LKDs in 2007 had no status or were not seen, vs. 11% in 2010. Few LKDs declined follow up (8% in 2007; 6% in 2010).
Program reporting for lab and clinical data ranged from 0% to 100% of LKDs, with a median of 10% in 2007, vs. 21% in 2010 (range: 0-100%). Program LDK volume was not associated with reporting, and some large and small programs achieved excellent rates of reporting.
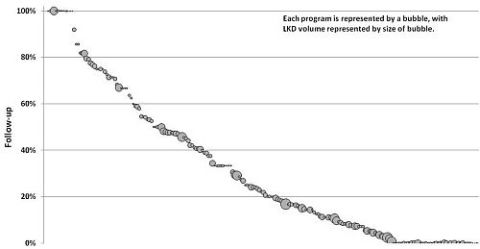
There has been a steady increase in follow-up reporting over the past several years. The small number of LKDs who declined follow-up shows that donor unwillingness may not be a major barrier. Program volume and reporting rates are unrelated, suggesting that continued improvements are achievable by large and small programs.
To cite this abstract in AMA style:
Wainright J, McBride M, Dew M, Bolton D, Thomas C. Follow-Up Reporting for Living Kidney Donors [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/follow-up-reporting-for-living-kidney-donors/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
