Extended Experience Using Tocilizumab (Anti-IL6R, TCZ) for the Treatment of Chronic Antibody Mediated Rejection (CABMR)
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Paris Translational Center for Organ Transplantation, Paris, France.
Meeting: 2018 American Transplant Congress
Abstract number: 206
Keywords: Alloantibodies, Highly-sensitized, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney Chronic Antibody Mediated Rejection
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Room 4B
Introduction: Disruption of the IL-6/IL-6R pathways may have benefits in treating allograft rejection. We reported our experience with anti-IL-6R (TCZ) as a treatment for CABMR (AJT 2017; 17: 2381-2389). CABMR is a complication of pre-existing donor specific antibodies (preDSAs) (Type 1) or de novo (dnDSAs) (Type 2). There are limited options for prevention & treatment of CABMR+TG. Patients who develop CABMR+TG have a poor prognosis with graft failure & return to dialysis. Here we report on our extended experience with TCZ for CABMR+TG. Methods: Since 4/2011 we identified 65 patients including those with CABMR+TG, DSA+ and/or AT1R ab+. TCZ treatment was pursued after other treatments had failed. Briefly, after diagnosis of CABMR, patients received TCZ 4-8mg/kg monthly for 3-37 doses & were followed up to 6 years from TCZ initiation. Patients were monitored for DSAs using Luminex, AT1Rab (ELISA), renal function, patient/graft survival and repeat biopsies (#11) by Banff criteria. Results: The mean time from transplant to TCZ treatment was 5.39±4.3 years & mean time from CABMR diagnosis to treatment was 1.37+1.62 years. Immunodominant (iDSA) levels tended to decrease after therapy (t0: 12,967±20000, t12M: 9180±6682,t36M: 3829±6001MFI) (p=NS). Mean eGFRs were 53.18±34.61ml/min at 0M vs. 50.43±36.37ml/min at 24M. Graft survival was compared to a standard (SOC) group (39-non concurrent CABMR patients) treated with IVIg + rituximab +/- PLEX. At 6 years, 92.6% of TCZ patients have functioning grafts v. 53.3% in SOC (p=0.0005). Two deaths in the TCZ group. Pre- & post-TCZ biopsies at mean of 29.5±18.7M from pre-biopsy showed significant reductions in g+ptc scores compared to biopsy at diagnosis. Conclusions: With extended follow-up, CABMR & TG patients treated with TCZ continue to show long term stabilization of renal function and graft survival in a population where poorer outcomes would be expected. 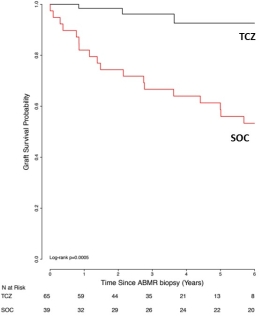
CITATION INFORMATION: Choi J., Aubert O., Louie S., Ammerman N., Vo A., Peng A., Huang E., Najjar R., Puliyanda D., Sethi S., Haas M., Lim K., Loupy A., Jordan S. Extended Experience Using Tocilizumab (Anti-IL6R, TCZ) for the Treatment of Chronic Antibody Mediated Rejection (CABMR) Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Choi J, Aubert O, Louie S, Ammerman N, Vo A, Peng A, Huang E, Najjar R, Puliyanda D, Sethi S, Haas M, Lim K, Loupy A, Jordan S. Extended Experience Using Tocilizumab (Anti-IL6R, TCZ) for the Treatment of Chronic Antibody Mediated Rejection (CABMR) [abstract]. https://atcmeetingabstracts.com/abstract/extended-experience-using-tocilizumab-anti-il6r-tcz-for-the-treatment-of-chronic-antibody-mediated-rejection-cabmr/. Accessed February 28, 2026.« Back to 2018 American Transplant Congress
