Experience with Obinutuzumab (Type II Anti-CD20) in Kidney Transplant Patients with Donor Specific Antibody (DSA+) Antibody Mediated Rejection.
Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
Meeting: 2017 American Transplant Congress
Abstract number: 293
Keywords: Kidney transplantation
Session Information
Session Name: Concurrent Session: Treatment of Antibody Mediated Rejection in Kidney Transplant Recipients
Session Type: Concurrent Session
Date: Monday, May 1, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: E354a
Introduciton:Rituximab is a type I chimeric IgG1 anti-CD20 antibody that has revolutionized the management and treatment of B-cell malignancies, and numerous autoimmune diseases. In addition, rituximab has become an essential component for the prevention and treatment of alloantibody mediated injury to allografts. Despite effectiveness, there are limitations of rituximab that often result in incomplete B-cell depletion and persistence of diseases. Newer approaches have focused on generating engineered anti-CD20 antibodies with enhanced functional activities that may lead to superior efficacy. Clinical data with the type II anti-CD20 (Obinutuzimab, [Obi], Roche-Genentech, South San Francisco, CA) indicate superior efficacy in treatment of B-cell malignancies (Mössner E, et al. Blood 2010. 115(22): p. 4393-402). Here, we explored the safety ability of Obinutuzimab to modify antibody-mediated allograft rejection (ABMR) and donor-specific antibodies (DSAs) in renal allograft recipients. Methods: For the past 15 months, 24 kidney transplant patients with biopsy proven, DSA+ ABMR who had failed treatment with IVIg + rituximab were treated with Obi 1gm x 1 dose. DSA levels, SCr, patient & graft survival as well as AE/SAE were monitored. Results: DSA results are shown in Fig. 1. Briefly, non-significant reductions were seen in HLA class I /and class II but dilutions were not assessed. Fig. 2 shows renal function over time was stable without significant improvement. Patient and graft survival at 1 year was 100%. From a safety standpoint, Obi was well tolerated w/o significant infusion related side-effects. One of the 24 patients had cold symptoms which developed one month after Obi however resolved without any treatment. Conclusions: From this relatively early experience with Obi in treatment of DSA+ ABMR, there appears to be an early effect on class I DSA reduction and stabilization of SCr. The drug appears to have a similar side-effect profile as rituximab, despite significantly better efficacy in B-cell reduction. Controlled studies are needed. 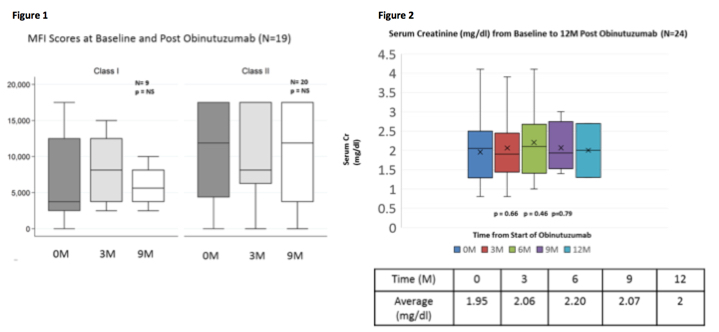
CITATION INFORMATION: Choi J, Vo A, Huang E, Louie S, Kang A, Peng A, Najjar R, Jordan S. Experience with Obinutuzumab (Type II Anti-CD20) in Kidney Transplant Patients with Donor Specific Antibody (DSA+) Antibody Mediated Rejection. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Choi J, Vo A, Huang E, Louie S, Kang A, Peng A, Najjar R, Jordan S. Experience with Obinutuzumab (Type II Anti-CD20) in Kidney Transplant Patients with Donor Specific Antibody (DSA+) Antibody Mediated Rejection. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/experience-with-obinutuzumab-type-ii-anti-cd20-in-kidney-transplant-patients-with-donor-specific-antibody-dsa-antibody-mediated-rejection/. Accessed March 9, 2026.« Back to 2017 American Transplant Congress
