Everolimus Facilitated Reduction of Tacrolimus in De Novo Liver Transplant Recipients: 12-24 Month Data from North America
Washington Univ School of Medicine, St Louis, MO
Columbia Univ Medical Center, New York, NY
Duke Univ Medical Center, Durham, NC
Medical Univ of SC, Charleston, SC
Univ of Medicine and Denstistry-NJ Medical School, Newark, NJ
Henry Ford Hosp, Detroit, MI
Novartis AG, Basel, Switzerland
Novartis Pharmaceuticals Corporation, East Hanover, NJ
Cleveland Clinic, Cleveland, OH
Meeting: 2013 American Transplant Congress
Abstract number: D1608
Purpose: In Study H2304, everolimus (EVR) facilitated early tacrolimus (TAC) minimization with comparable efficacy and better renal function vs standard TAC at 12 months (M) after liver transplantation (LTx) (De Simone, 2012). At randomization (RDN), in North America (NA) vs other regions, mean Model for End-stage Liver Disease (MELD) score was higher (22.7 vs 17.6 in Europe/18.5 in rest of world) and estimated glomerular filtration rate (eGFR) lower (76 vs 84/79 mL/min/1.73 m2), and there was a higher incidence of both diabetes and hepatitis C virus. Here we report 12 and 24M results for the NA subpopulation.
Method: In this 24M, multicenter, open-label study, 211 de novo LTx recipients from NA were randomized after a 30-day run-in period with TAC±mycophenolic acid to receive either EVR (C0 3-8 ng/mL)+reduced TAC (C0 3-5 ng/mL; EVR+rTAC, n=65), EVR (C0 6-10 ng/mL)+TAC withdrawal at M4 (TAC-WD, n=68), or standard TAC (C0 6-10 ng/mL; TAC-C, n=78); all with steroids. Primary endpoint at M12 (amended protocol) was the composite efficacy failure rate of treated biopsy proven acute rejection (tBPAR), graft loss, or death.
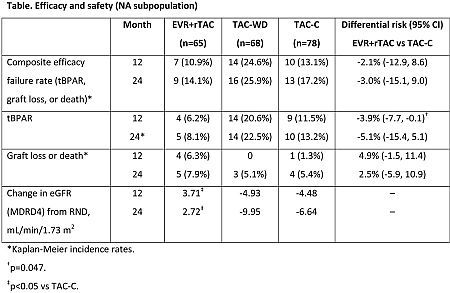
Results: Enrollment in the TAC-WD arm was prematurely terminated due to a higher rate of tBPAR. Efficacy results were similar at 12 and 24M with improved renal function in the EVR+rTAC vs TAC-C group (table). Incidence rates of adverse events (AEs)/infections and discontinuations due to AEs were similar between EVR+rTAC (96.9% and 18.5%) and TAC-C (94.9% and 14.1%).
Conclusions: In patients from NA, EVR facilitated TAC reduction from M1 showed comparable efficacy and improved renal function over standard exposure TAC.
Brown Jr, R.: Grant/Research Support, Novartis. Sudan, D.: Other, Novartis, Medical Consultant-Liver Advisory Board. Huang, M.: Speaker’s Bureau, Vertex, Other, Bayer-Onyx, Advisory Board Member, Gilead, Advisory Board Member. Junge, G.: Employee, Novartis. Hexham, J.: Employee, Novartis. Dong, G.: Employee, Novartis. Wiland, A.: Employee, Novartis. Fung, J.: Other, Vital Therapies, Consultant.
To cite this abstract in AMA style:
Chapman W, Jr RBrown, Sudan D, Chavin K, Koneru B, Huang M, Junge G, Hexham J, Dong G, Wiland A, Fung J. Everolimus Facilitated Reduction of Tacrolimus in De Novo Liver Transplant Recipients: 12-24 Month Data from North America [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/everolimus-facilitated-reduction-of-tacrolimus-in-de-novo-liver-transplant-recipients-12-24-month-data-from-north-america/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
