Evaluation of Optimization of Workflow for Timing of Inpatient Therapeutic Drug Monitoring of Maintenance Immunosuppressive Drugs in Abdominal Transplant Recipients
Department of Pharmacy, Northwestern Memorial Hospital, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: B-215
Keywords: Immunosuppression, Kidney/liver transplantation, Monitoring, Pharmacokinetics
Session Information
Session Name: Poster Session B: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Therapeutic drug monitoring (TDM) is essential to the safety and efficacy of immunosuppressants (IS) given their narrow therapeutic index. After an internal review, it was found that TDM samples of IS were drawn early and with high variability, leading to inaccurate intervals between administration time and collection time resulting in a low number of appropriate troughs. As a result, workflow was optimized between pharmacists, phlebotomists, and nurses to modify lab draw times and maintain administration times. This analysis evaluates the appropriateness of lab draws for the TDM of IS agents after optimization of the timing of lab draws and to assess the change in accuracy of trough concentrations obtained post-workflow refinement.
*Methods: Evaluation of IS draws collected on the medical surgical transplant nephrology/hepatology floor associated with a singular admission during pre-implementation (Jun ’18 – Aug ’18) were compared to post-implementation (Mar ’19 – May ’19). Optimization of workflow included modifying lab draw times from 0200 – 0400 to 0500 – 0600, while maintaining the same IS administration times of 0600 and 1800. Accurate troughs were defined based on clinical relevance as 11-13 hours for drugs requiring 12-hour trough and 22-26 hours for drugs requiring 24-hour trough assessments.
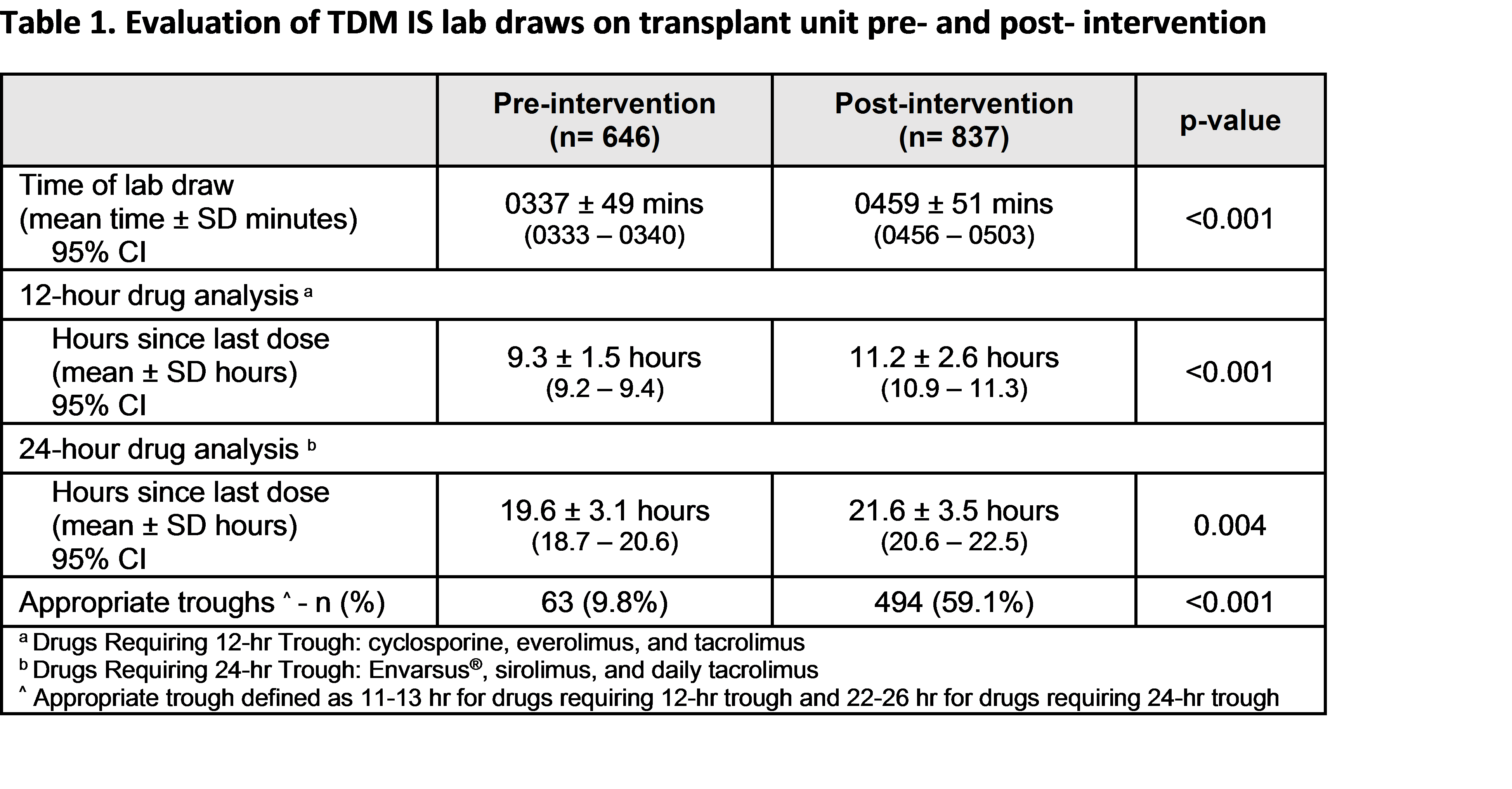
*Results: A total of 1483 lab draws were assessed (646 pre-implementation, 837 post-implementation). The mean time of lab draw was later post-implementation (0459 ± 51 mins), which correlated with more desired trough hour assessments for both 12-hour agents (9.3 ± 1.5 hr vs. 11.2 ± 2.6 hr) and 24-hour agents (19.6 ± 3.1hr vs. 21.6 ± 3.5hr). The modification in workflow resulted in a significantly greater percentage of accurate troughs (9.8% vs. 59.1%, p <0.001).
*Conclusions: The change in workflow to modify targeted timing of pre-dose trough lab draws was significantly associated with a greater number of accurate trough levels. This collaboration helped to optimize the interpretation of accurate IS drug levels for appropriate dose adjustments of narrow therapeutic index immunosuppressive agents.
To cite this abstract in AMA style:
Schulte J, Mehta S, Vargas E, Cunningham K. Evaluation of Optimization of Workflow for Timing of Inpatient Therapeutic Drug Monitoring of Maintenance Immunosuppressive Drugs in Abdominal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-optimization-of-workflow-for-timing-of-inpatient-therapeutic-drug-monitoring-of-maintenance-immunosuppressive-drugs-in-abdominal-transplant-recipients/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress