Evaluation of Hepatitis C Positive Donor to Hepatitis C Negative Recipient Kidney Transplant in a Highly Sensitized Patient Population
A. T. Iaria, M. Martin, A. Hietpas, I. Tang, S. Koppe, I. Tzvetanov, E. Benedetti, C. Muran
University of Illinois at Chicago, Chicago, IL
Meeting: 2022 American Transplant Congress
Abstract number: 556
Keywords: Hepatitis C, High-risk, Highly-sensitized, Kidney transplantation
Topic: Clinical Science » Infection Disease » 27 - Non-Organ Specific: Viral Hepatitis
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:00pm-6:10pm
Presentation Time: 6:00pm-6:10pm
Location: Hynes Room 312
*Purpose: This study assessed outcomes of hepatitis C virus (HCV) donor positive to recipient negative (D+/R-) kidney transplants (KT) in high immunologic risk patients. Literature reports positive short-term outcomes in low immunologic risk patients, but limited data exists to support HCV D+/R- KT in high immunologic risk patients.
*Methods: This retrospective cohort study included HCV antibody negative (-) recipients of a nucleic acid test (NAT) positive (+) KT who received HCV treatment with direct-acting antiviral therapy from 12/1/20 and 11/30/21. NAT+ KT recipients were matched 1:1 with NAT – KT recipients based on age, body mass index, and gender. All serologies were confirmed prior to study inclusion. The primary outcome was a composite of patient and graft survival 3 months post-KT. Secondary outcomes included percent of patients with sustained virologic response (SVR), time to HCV treatment initiation, incidence of cytomegalovirus (CMV) and BK viremia, presence of de novo donor specific antibody (DSA), rejection and HCV treatment related adverse drug reactions (ADRs). Descriptive statistics were used for baseline characteristics and a Log-Rank test was used for the primary outcome.
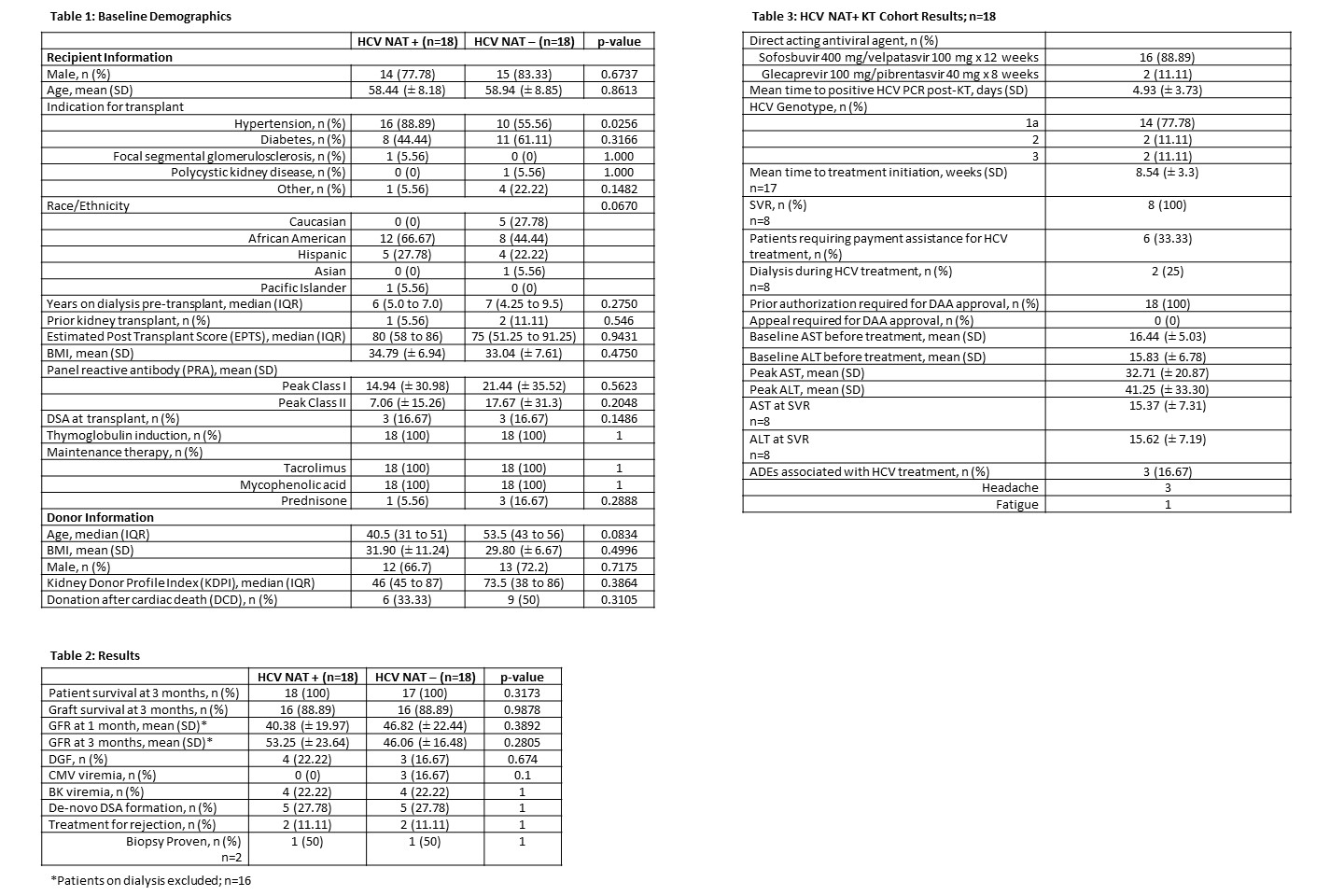
*Results: Eighteen HCV NAT+ KT recipients were matched with 18 HCV NAT- KT recipients. Study population was similar between groups and had end stage renal disease due to diabetes and/or hypertension, mean class I PRA >14%, pre-transplant DSA in 16% of patients in each cohort, and all received induction with rabbit anti-thymocyte globulin (Table 1). There was no difference in patient (p=0.32) and graft survival (p=0.99) between groups at 3 months post-KT. One death due to COVID-19 occurred in the NAT- group. There was no difference in estimated glomerular filtration rate (eGFR) at 1 (p=0.39) and 3 months (p=0.28), incidence of delayed graft function (DGF) (p= 0.67) or CMV (p=0.1) and BK viremia (p=1) between groups. (Table 2). HCV transmission occurred in all NAT+ KT recipients, and all who completed therapy achieved SVR. Treatment was initiated on average 8.5 weeks post-KT (Table 3). Notably, 33% of patients required financial assistance to obtain HCV treatment.
*Conclusions: Use of HCV D+/R- KT resulted in no difference in patient and graft survival at 3 months in this matched cohort. HCV NAT + KT patients should be connected with financial assistance programs early to promote timely treatment initiation.
To cite this abstract in AMA style:
Iaria AT, Martin M, Hietpas A, Tang I, Koppe S, Tzvetanov I, Benedetti E, Muran C. Evaluation of Hepatitis C Positive Donor to Hepatitis C Negative Recipient Kidney Transplant in a Highly Sensitized Patient Population [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-hepatitis-c-positive-donor-to-hepatitis-c-negative-recipient-kidney-transplant-in-a-highly-sensitized-patient-population/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress