Effect of Glecaprevir/Pibrentasvir on Weight-Adjusted Tacrolimus Trough/Dose Ratios in Heart and Kidney Recipients
1Pharmacy, Montefiore Medical Center, Bronx, NY, 2Medicine, Montefiore Medical Center, Bronx, NY, 3Division of Cardiology, Department of Medicine, Montefiore Medical Center, Bronx, NY, 4Cardiothoracic Surgery, Montefiore Medical Center, Bronx, NY, 5Surgery, Montefiore Medical Center, Bronx, NY
Meeting: 2020 American Transplant Congress
Abstract number: 607
Keywords: FK506, Heart transplant patients, Hepatitis C, Kidney transplantation
Session Information
Session Name: All Organs: Pharmacogenomics / Pharmacokinetics
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: An innovative strategy to meet the demands of patients requiring heart or kidney transplantation is the acceptance of hepatitis C viremic organs. Direct acting antivirals (DAAs) are highly effective at curing HCV infection, but the pharmacokinetic implications of DAA use on calcineurin inhibitor therapy post-transplant are unknown. We sought to investigate the effects of glecaprevir/pibrentasvir (G/P), a CYP3A4 substrate and inhibitor, on weight-adjusted tacrolimus (FK) trough/dose (T/D) ratio following isolated heart or kidney transplantation.
*Methods: This was a single-center, retrospective analysis of HCV negative heart or kidney transplant recipients who accepted HCV viremic organs followed by 12-weeks of G/P therapy. Patients received standard triple maintenance immunosuppression with FK, mycophenolate and prednisone. Those initiated on concomitant CYP3A inducers/inhibitors were excluded. Weight-normalized T/D ratio was assessed while patients were on stable doses of FK to achieve steady-state prior to, during, and after G/P treatment. Steady-state was defined by receiving a stable dose of FK without needing adjustment to maintain target trough for 2-3 days.
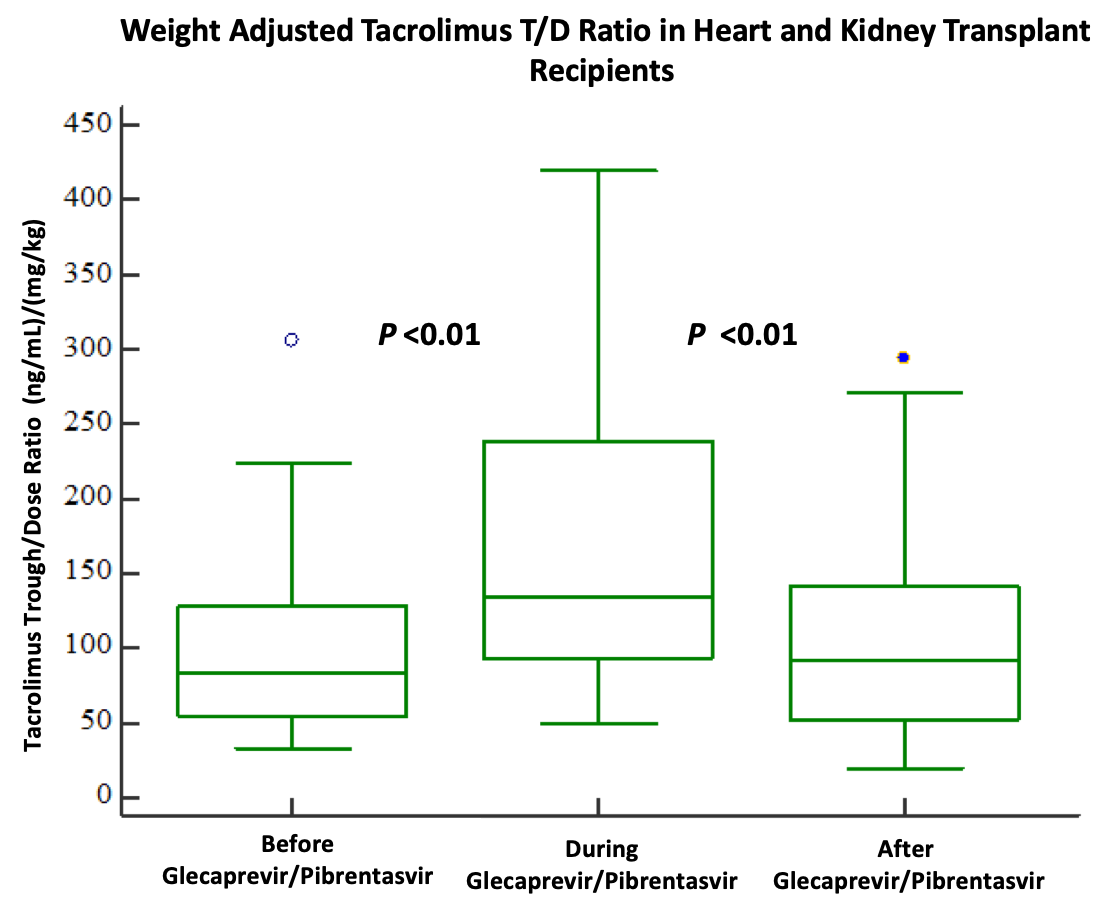
*Results: Twenty-seven HCV negative organ recipients were evaluated. Median age was 70 years (IQR 66-71). The weight-normalized T/D ratio was greater during G/P treatment (134, IQR 101-195) compared to before treatment (83, IQR 54-129) (P<0.01), but lower after completion of treatment (92, IQR 57-112) compared to during treatment (134, IQR 101-195)(P<0.01). Four patients (3 kidney, 1 heart) experienced acute rejection.
*Conclusions: Initiation of glecaprevir/pibrentasvir in heart or kidney transplant recipients induces a reversible change in tacrolimus metabolism. Based on the results of our study, a 40-50% tacrolimus dose reduction may be considered at the time of glecaprevir/pibrentasvir initiation. No clear relationship of HCV viremic organ transplantation and rejection risk was found. Larger studies are warranted to validate these findings.
To cite this abstract in AMA style:
Nnani D, Campbell A, Ajaimy M, Patel SR, Saeed O, Goldstein DJ, Graham J, Jorde UP. Effect of Glecaprevir/Pibrentasvir on Weight-Adjusted Tacrolimus Trough/Dose Ratios in Heart and Kidney Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/effect-of-glecaprevir-pibrentasvir-on-weight-adjusted-tacrolimus-trough-dose-ratios-in-heart-and-kidney-recipients/. Accessed March 3, 2026.« Back to 2020 American Transplant Congress