Early Tacrolimus Trough Attainment in De Novo Kidney Transplant Recipients Treated with LCP-Tacrolimus (LCPT) vs. Immediate-Release Tacrolimus (IR-Tac)
1Charité University Medicine Berlin, Berlin, Germany, 2UCLA Medical Center, Los Angeles, CA, 3Veloxis Pharmaceuticals Inc., Cary, NC, 4University Hospital of Münster, Münster, Germany
Meeting: 2019 American Transplant Congress
Abstract number: A247
Keywords: Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
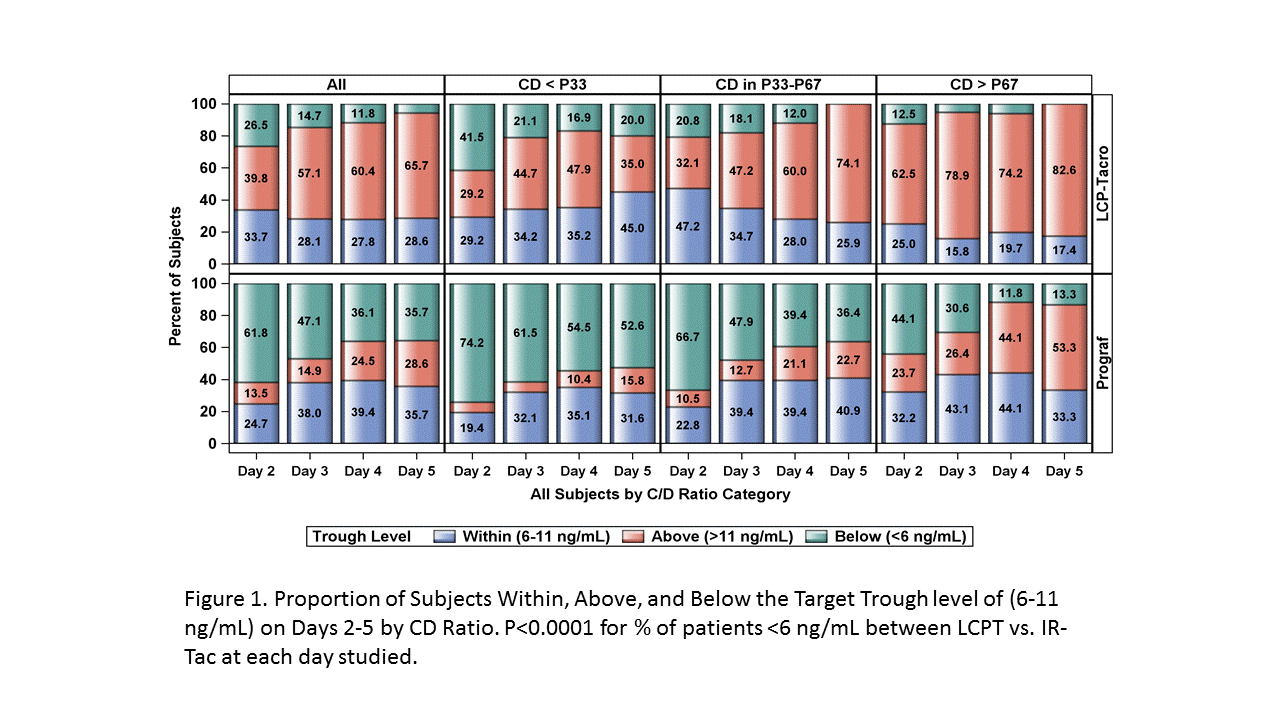
*Purpose: Early achievement of therapeutic tacrolimus concentrations has been associated with reduced rates of acute rejection. A prospective, randomized, controlled study demonstrated safe and efficacious use of LCPT in de novo kidney transplantation. The purpose of this analysis is to evaluate trough level attainment in the immediate post-transplant setting for LCPT vs IR-Tac.
*Methods: A post-hoc analysis of a Phase III clinical trial was conducted. Within 48 hours of transplantation patients were randomized to receive LCPT starting at 0.17 mg/kg/day or IR-Tac 0.1 mg/kg/day. Subsequent doses were adjusted to maintain target trough levels of 6-11ng/mL for the first 30 days. Patients were divided into low, medium and high CD ratio (calculated at 1 month) to estimate rapid, intermediate, and slow metabolizers, respectively. The proportion of patients below, within, and above target range were determined for days 2-5 post-tacrolimus initiation.
*Results: A total of 543 patients were randomized in the study. Proportion of patients below, within, and above target range are shown for all patients and by CD ratio group in Figure 1. After initial dosing, 24.7% of IR-Tac patients were within target range, with a predominance of patients (61.8%) being below range. While 33.7% of LCPT patients were within range after initial dosing, more patients demonstrated concentrations above target range (39.8%). A similar pattern was seen across intermediate (P33-P67) and slow (CD>P67) metabolizers. Of note, in rapid metabolizers (CD
To cite this abstract in AMA style:
Budde K, Bunnapradist S, Patel SJ, Stevens DR, Meier-Kriesche U, Suwelack B. Early Tacrolimus Trough Attainment in De Novo Kidney Transplant Recipients Treated with LCP-Tacrolimus (LCPT) vs. Immediate-Release Tacrolimus (IR-Tac) [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/early-tacrolimus-trough-attainment-in-de-novo-kidney-transplant-recipients-treated-with-lcp-tacrolimus-lcpt-vs-immediate-release-tacrolimus-ir-tac/. Accessed March 4, 2026.« Back to 2019 American Transplant Congress