Donors with Isolated Anti-HBc (“HBcAb Only”) Antibodies: True Evidence for Past Infection or Laboratory Artifact? Toward a Simple Confirmatory Test for HBcAb.
1Mendez National Institute of Transplantation Foundation, Los Angeles, CA
2Viracor-IBT Laboratories, Los Angeles, CA
3Western University of Health Sciences, Pomona, CA.
Meeting: 2016 American Transplant Congress
Abstract number: 71
Keywords: Cadaveric organs, Hepatitis B, Safety, Screening
Session Information
Session Name: Concurrent Session: SOT: HIV, HBV, & HCV
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Room 313
Use of organs from donors positive for hepatitis B virus (HBV) markers may safely expand the donor pool. Recently the American Society of Transplantation expert panel recommended that organs from “HBcAb only” donors be considered for transplantation with antiviral HBV prophylaxis (AJT, May, 2015). The HBV prophylaxis is a long-term treatment, with side effects, and is expensive. However, based upon epidemiological and vaccine studies it was determined that for 50% of these individuals HBcAb results could be false positive and only could be resolved with the elaborate algorithms. These algorithms are not feasible for organ donor testing, and there is no commercial confirmatory test for HBcAb.
Aim: To develop a supplemental assay for resolving questionable HBcAb+ cases among organ donors.
Methods: To develop the HBcAb neutralization assay, we used WHO HBcAb standard, recombinant HBcAg, and recombinant HCV core antigen as specificity control. Tested serum was incubated 1:20 with rHBcAg or rHCcAg or mock buffer for 1hr/37oC, and tested using HBcAb screening assay. Neutralization ≥ 50% was considered significant. 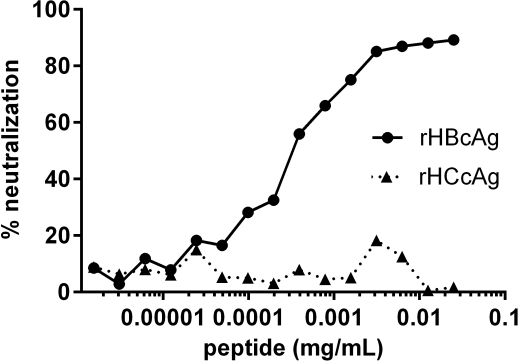
Results: Our neutralization protocol was reproducible, robust, and showed specific inhibition of HBcAb (figure). When applied to well-characterized specimens from donors, sera with weak HBsAb reactivity or with HBcAb as the only marker showed no neutralization. In contrast, almost all donors with strong HBcAb and HBsAb co-reactivity showed significant neutralization.
Conclusions: We developed a simple neutralization procedure to confirm the specificity of HBcAb. Our preliminary data suggests that significant proportion of “HBcAb only” donors could be false positive. Inclusion of the HBcAb neutralization test in testing protocol could help resolve questionable HBcAb, prevent unnecessary post-transplant preventive anti-HBV treatment, and contribute to better organ utilization.
CITATION INFORMATION: Chang Y, Chinchilla-Reyes C, Koss M, Nowicki M. Donors with Isolated Anti-HBc (“HBcAb Only”) Antibodies: True Evidence for Past Infection or Laboratory Artifact? Toward a Simple Confirmatory Test for HBcAb. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Chang Y, Chinchilla-Reyes C, Koss M, Nowicki M. Donors with Isolated Anti-HBc (“HBcAb Only”) Antibodies: True Evidence for Past Infection or Laboratory Artifact? Toward a Simple Confirmatory Test for HBcAb. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/donors-with-isolated-anti-hbc-hbcab-only-antibodies-true-evidence-for-past-infection-or-laboratory-artifact-toward-a-simple-confirmatory-test-for-hbcab/. Accessed March 4, 2026.« Back to 2016 American Transplant Congress
