Discontinuation Rates in Multicenter Trials – Are They Driven by Attitudes or Facts?
1Athena and Hephaistos, Study Group, Germany, 2Hephaistos, Study Group, Germany, 3Athena, Study Group, Germany, 4Novartis Pharma GmbH, Nürnberg, Germany
Meeting: 2020 American Transplant Congress
Abstract number: B-107
Keywords: Immunosuppression, Kidney/liver transplantation, Outcome
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The ATHENA [NCT01843348] and Hephaistos [NCT01551212] trials were designed to compare everolimus [EVR] in combination with reduced tacrolimus [rTAC] or reduced cyclosporine A [rCyA] vs. a standard regimen of mycophenolic acid [MPA] and TAC in de novo kidney transplant [KTx] recipients, and EVR plus rTAC vs. TAC alone in de novo liver transplant [LTx] recipients, respectively. As it has often been claimed that treatment discontinuation rates due to AEs are higher with EVR than with conventional regimes, discontinuation rates in these 2 studies were analyzed.
*Methods: Both studies were 12M prospective, open-label multicenter trials. ATHENA included 612 patients [pts] in Germany and France, randomized at time of KTx to EVR/rTAC, EVR/rCyA or TAC/MPA. In HEPHAISTOS, 333 pts were randomized between day 7-21 after LTx to EVR/rTAC or TAC alone, all with steroids until M6.
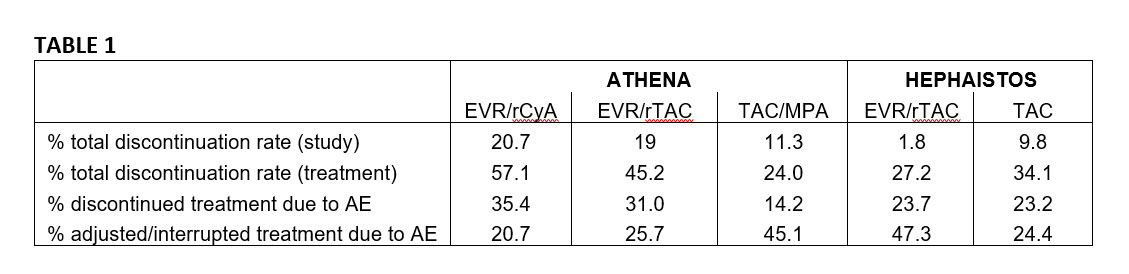
*Results: All discontinuation and adjustment rates are shown in Table 1. No AE-specific correlation was detected for either, indicating no specific drug related reason for discontinuation or adjustment. Interestingly, the incidence of total discontinuation rates was contrary between EVR and control arms in both studies. Moreover, rates of study drug discontinuation in ATHENA varied widely between centers (0-100%, 0-100% and 0-60% in the EVR/rCyA, EVR/rTAC and TAC/MPA groups, respectively), while variations were less in Hephaistos (0-53% EVR/TAC and 0-62% TAC group).
*Conclusions: Both, ATHENA and HEPHAISTOS, confirmed the safe and effective use of EVR with rTAC or rCyA in KTx and LTx. However, the most striking finding of this analysis was the inverse incidence of discontinuation rate in control vs treatment arms between the KTx and LTx studies. Further, AE analysis revealed no specific drug related reasons for discontinuations in general, yet showed significant differences between not only organs but also centers. These interesting findings are so far unexplained, but we hypothesize, that attitudes driven by investigators’ background and/or experience play a role and this might aid in understanding the results and differences observed in multicenter clinical trials.
To cite this abstract in AMA style:
Nashan B, Braun F, Dragun D, Hauser IA, Schemmer P, Schlitt HJ, Sommerer C, Suwelack B, Schiedel C, Kroeger I, Thaiss F. Discontinuation Rates in Multicenter Trials – Are They Driven by Attitudes or Facts? [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/discontinuation-rates-in-multicenter-trials-are-they-driven-by-attitudes-or-facts/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress