Development of a Clinically Relevant Conditioning Regimen Using Available Costimulatory Blockade Reagents for Induction of Renal Allograft Tolerance Via Mixed Hematopoietic Chimerism
Transplant Center, Massachusetts General Hospital, Boston, MA
Meeting: 2013 American Transplant Congress
Abstract number: 242
Background: We have previously reported that costimulation blockade (CB) with anti-CD154 mAb (mAb154) promotes mixed chimerism and renal allograft tolerance in nonhuman primates. However, this approach has not been clinically applicable because of the thrombogenic effects associated with mAb154 treatment. In this study, we investigated whether clinically available CTLA4Ig fusion proteins (abatacept or belatacept) could be substituted for mAb154 to achieve renal allograft tolerance using our mixed chimerism approach.
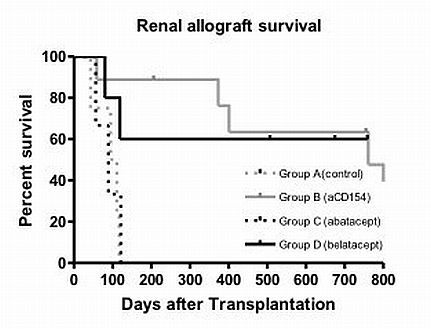
Method: Cynomolgus monkeys were conditioned with low dose total body irradiation, thymic irradiation, ATGAM and received an MHC-mismatched kidney and bone marrow cells from the same donor. CB was administered during the first 2 weeks. Cyclosporine was also administered for 28 days, after which no immunosuppression (IS) was given. Recipients were divided into four groups; Group A (no CB, n=4), B (mAb154, n=9), C (abatacept, n=3) and D (belatacept, n=5).
Results: Without CB, all recipients rejected their renal allografts after discontinuation of IS. With mAb154 (Group B), all recipients developed transient multilineage chimerism and seven survived > 300 days without Immunosuppression (IS). In Group C (abatacept), 2/3 recipients developed chimerism, but all rejected their kidney allografts after IS cessation (days 56, 89, 121). In contrast, in Group D (belatacept), 3/5 animals developed mixed chimerism and have continued renal allograft survival at > 500 days without IS (day >760, >676, >508). Two recipients with poor chimerism in this group had prolonged survival but rejected their renal allografts after IS was discontinued.
Conclusion: CB with belatacept promotes mixed chimerism and renal allograft tolerance in NHPs and appears to represent a promising agent for clinical trials of tolerance induction in renal allograft recipients.
To cite this abstract in AMA style:
Yamada Y, Lee S, Boskovic S, Nadazdin O, Smith R, Colvin R, Madsen J, Sachs D, Benichou G, Cosimi B, Kawai T. Development of a Clinically Relevant Conditioning Regimen Using Available Costimulatory Blockade Reagents for Induction of Renal Allograft Tolerance Via Mixed Hematopoietic Chimerism [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/development-of-a-clinically-relevant-conditioning-regimen-using-available-costimulatory-blockade-reagents-for-induction-of-renal-allograft-tolerance-via-mixed-hematopoietic-chimerism/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
