Defect in the Th17 Pathway Worsens Renal Interstitial Fibrosis and Tubular Atrophy after Ischemic Acute Kidney Injury
Multi-Organ Transplant Program, McGill University Health Centre, Montreal, QC, Canada
Department of Pathology, McGill University, Montreal, QC, Canada
Meeting: 2013 American Transplant Congress
Abstract number: C1188
Background: Ischemic acute kidney injury (AKI), which is inherent to kidney transplantation, is associated with the detrimental development of interstitial fibrosis/tubular atrophy (IF/TA). IL-17A, the signature cytokine of the Th17 cell, is fibrogenic in models of heart and lung transplantation. It also promotes acute damage after ischemic AKI, but its implication in the progression to renal IF/TA is unknown.
Methods: Wild-type (WT), IL-17A KO, and BATF KO (Th17 lineage deficient) mice on a C57BL/6 background underwent unilateral renal pedicle clamping for 30 minutes, followed by reperfusion for up to 42 days. Injured (IK) and control (CK) kidneys were digested in collagenase, and lymphocytes isolated by density gradient. Phenotypic analysis of Th17, Th1, Th2, and Treg cells was performed by flow cytometry. IF/TA was quantified by a blinded pathologist on H&E and Masson’s Trichrome kidney sections.
Results: CD4+IL-17A+ Th17 cells progressively infiltrated the IK but not the CK in WT mice, peaking at 14 days after reperfusion (Fig. 1A). The majority co-expressed the Th17-specific transcription factor RORgt, and co-secreted IL-17F. At 42 days after reperfusion, significant IF/TA developed in the IK of WT mice, while the CK was IF/TA-free. In comparison to WT mice, IL-17A KO had worse IF/TA, while deficiency in both IL-17A and F (BATF KO) worsened tubular atrophy only (Fig. 1B-C). IL-17F alone did not explain worse fibrosis as its expression in IL-17A KO was reduced compared to WT mice. There was no difference in the expression of IFN-Γ (Th1), IL-4 (Th2), or FoxP3 (Treg) by kidney-infiltrating CD4+ T cells after reperfusion between WT and KO mice.
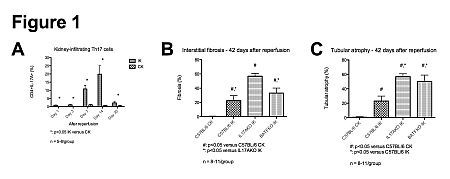
Conclusion: Following ischemia-reperfusion injury, there is a long-lasting infiltration of Th17 cells in the IK. Contrary to previous evidence in heart and lung models, a defect in the Th17 pathway is fibrogenic after renal AKI and worsens tubular atrophy. Targeting the Th17 pathway to reduce acute damage after AKI could worsen chronic injury.
To cite this abstract in AMA style:
Nguyen M, Michel R, Fryml E, Sahakian S, Liu S, Tchervenkov J, Paraskevas S. Defect in the Th17 Pathway Worsens Renal Interstitial Fibrosis and Tubular Atrophy after Ischemic Acute Kidney Injury [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/defect-in-the-th17-pathway-worsens-renal-interstitial-fibrosis-and-tubular-atrophy-after-ischemic-acute-kidney-injury/. Accessed February 27, 2026.« Back to 2013 American Transplant Congress
