Decision-Making, Informed Consent, and the Experiences of Recipients of Experimental HCV Positive-to-Negative Renal Transplants
JHU, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: D159
Keywords: Hepatitis C, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Infectious
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: The safety and efficacy of transplanting HCV-infected (HCV+) donor organs into HCV-uninfected recipients (HCV D+/R-) is being studied. Recipient experiences are essential to comprehensively assessing the risks and benefits of such transplants.
Methods: EXPANDER is a pilot clinical trial (NCT02781649) of HCV D+/R- transplants with direct-acting antivirals as pre- and post-kidney transplant (KT) prophylaxis. We interviewed EXPANDER participants within 2 weeks post-KT and again 3 months post-KT. Interviews addressed decision-making, informed consent, and overall experience in the trial (N=8/10, response rate 80%). Interviews were recorded and transcribed. Qualitative themes were inductively derived from interviews and independently applied and reconciled by 2 coders.
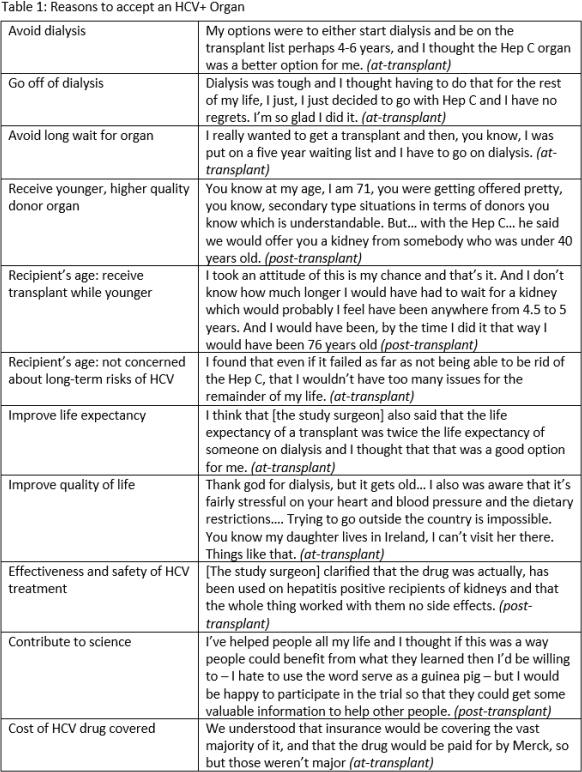
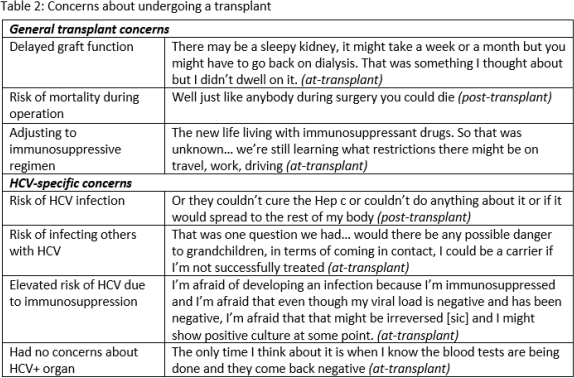
Results: While participants had some concerns about receiving an HCV+ organ, they decided to accept one based on their assessments of risks and benefits; they believed the primary benefit was discontinuing or avoiding dialysis (Tables 1, 2). They had very optimistic expectations about the likelihood of success at the time of enrollment. All participants recalled providing informed consent; they reported the process was thorough and that all concerns were addressed. Participants did not regret receiving an experimental HCV D+/R- KT. Responses did not change substantially from the at-KT to post-KT interviews.
Discussion: Participants recognized the risks and benefits of experimental HCV D+/R- transplants and did not regret participating. Given the unknowns of this experimental intervention, their optimism about the likelihood of success suggests potential therapeutic misconception. Nevertheless, these experiences contribute to a robust assessment of the appropriateness of further research on HCV D+/R- transplants.
CITATION INFORMATION: Rasmussen S., Seaman S., Brown D., Desai N., Sulkowski M., Segev D., Sugarman J., Durand C. Decision-Making, Informed Consent, and the Experiences of Recipients of Experimental HCV Positive-to-Negative Renal Transplants Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Rasmussen S, Seaman S, Brown D, Desai N, Sulkowski M, Segev D, Sugarman J, Durand C. Decision-Making, Informed Consent, and the Experiences of Recipients of Experimental HCV Positive-to-Negative Renal Transplants [abstract]. https://atcmeetingabstracts.com/abstract/decision-making-informed-consent-and-the-experiences-of-recipients-of-experimental-hcv-positive-to-negative-renal-transplants/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress