De Novo Use of Envarsus (LCPT) in African American and Elderly Kidney Transplant Recipients: An Early Single Center Experience
1Department of Pharmacy, Vanderbilt University Medical Center, Nashville, TN, 2Department of Surgery, Vanderbilt University Medical Center, Nashville, TN, 3Vanderbilt Transplant Center, Vanderbilt University Medical Center, Nashville, TN, 4Department of Medicine, Vanderbilt University Medical Center, Nashville, TN
Meeting: 2020 American Transplant Congress
Abstract number: C-119
Keywords: African-American, Elderly patients, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Clinical trials with LCPT have shown a steadier and more consistent concentration time profile over 24 hours with increased bioavailability compared to twice daily tacrolimus. Data also indicate that the pharmacokinetics of LCPT is less influenced by pharmacogenomic variances in African American (AA) patients, and it may be associated with reduced tacrolimus-induced neurological effects. We report a retrospective review of short term outcomes in AA and elderly adults receiving LCPT de novo following kidney transplantation.
*Methods: Patient demographics, transplant-related characteristics, adverse events, and longitudinal tacrolimus levels, doses and adjustments as well as renal function were recorded. Elderly was defined as greater than 65 years. The starting dose of LCPT was 6 or 8 mg every 24 hours as per provider preference. Data were analyzed using summary statistics and mixed effects models of longitudinal data.
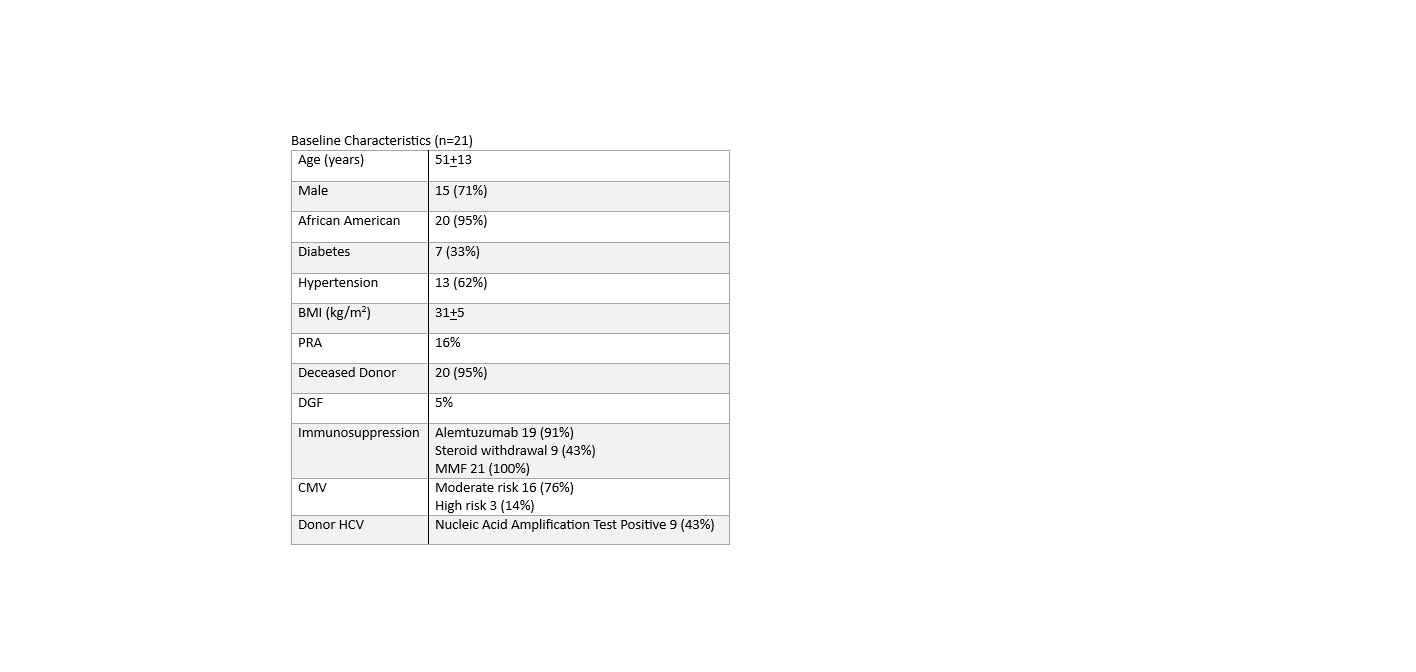
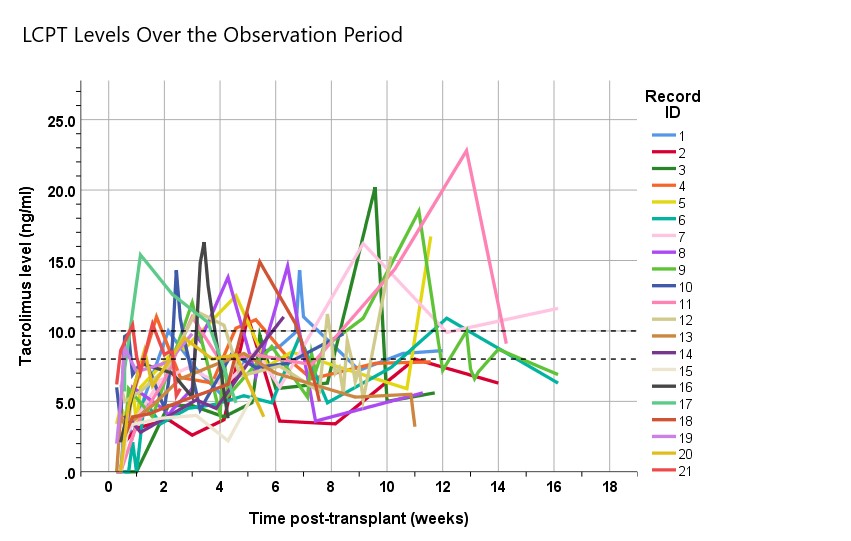
*Results: Baseline characteristics are listed in the table. Follow-up averaged 10±4 weeks (range 3-16). 20 of 21 patients (95%) achieved tacrolimus levels within the target range (8-10 ng/ml) at least once for weighted average therapeutic doses of 9±1 mg (0.1±0.01 mg/kg). Time to the 1st therapeutic level was 3.8+0.6 weeks. Tacrolimus levels ranged from 4.5 to 14.1 ng/ml over the reporting period (Figure). Overall adverse events included 14 readmissions, 5 infections, and 4 with new onset tremor. Renal function improved significantly over time as expected after transplantation.
*Conclusions: Our early experience demonstrates safe use of de novo LCPT in this subset of patients.
To cite this abstract in AMA style:
Wilson N, Leek R, Forbes R, Shaffer D, Rega S, Feurer I, Concepcion B, Langone A. De Novo Use of Envarsus (LCPT) in African American and Elderly Kidney Transplant Recipients: An Early Single Center Experience [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-use-of-envarsus-lcpt-in-african-american-and-elderly-kidney-transplant-recipients-an-early-single-center-experience/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress