De Novo DSA against Third Party Extension Vessels Used in Kidney and Pancreas Transplantation
University of Wisconsin, Madison.
Meeting: 2018 American Transplant Congress
Abstract number: C27
Keywords: Alloantibodies, Kidney/pancreas transplantation, Renal artery stenosis, Risk factors
Session Information
Session Name: Poster Session C: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Third party ABO-compatible vessel allografts (VA) are sometimes used as extension vessels for kidney and pancreas transplants (TX). The frequency and implications of de novo DSA (dnDSA) to these VA are not known.
We reviewed 44 TX requiring third party VA performed at our program between 1/2011 and 6/2014. Patients excluded were: 3 for whom VA HLA typing was unavailable, 2 zero-mismatch TX who were not tested for post-TX DSA and 1 with immediate graft thrombosis. Primary outcome was development of anti-VA dnDSA. Cox-regression analysis was performed to identify predictors for their development.
76.3% of the recipients were men, 80% were Caucasian and mean age was 53.6±13.4 yrs. 92.1% of VA were used for kidney TX (44.7% arterial, 39.5% venous, 7.9% both) and 7.9% for pancreas TX (all arterial). All were on calcineurin inhibitors and 95% on steroids at 1-month post-TX. Anti-VA dnDSA developed in 8 (21%) patients after a median of 378 days (range 7–1548 days). 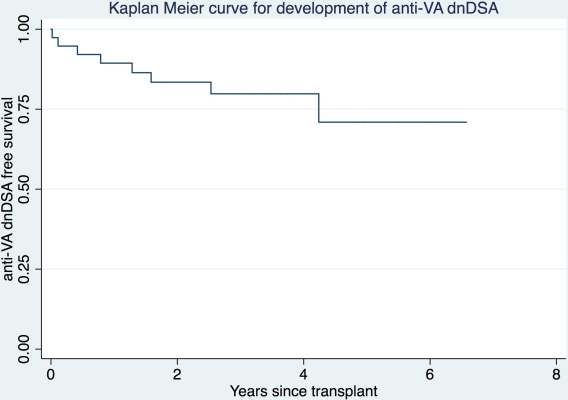 25% had class I DSA, 37.5% had class II DSA and 37.5% had both. Median sum MFI was 6219 (range 1223–124449). Compared to recipients that did not develop anti-VA dnDSA, those who did were younger (45.7 vs 55.7 yrs), had more HLA mismatches with the vessel donor (87.5% vs 63.3% had ≥ 6/8, 6 being the median for the entire group), were more likely to have developed anti-organ dnDSA (25.0% vs 10%) and have DGF (25.0% vs 10%). Induction regimens, PRA, repeat TX, living vs deceased TX, pre-TX anti-organ and anti-VA DSA, and race and gender distributions were similar between the two groups. Cox regression modeling revealed hazard ratios of 9 (p=0.07) and 4 (p=0.18) for development of anti-VA dnDSA with higher number of HLA mismatches with vessel donor and anti-organ dnDSA respectively. One patient was diagnosed with TX renal artery stenosis 3.2 years after anti-VA dnDSA detection; no patient without anti-VA dnDSA suffered any vascular complications.
25% had class I DSA, 37.5% had class II DSA and 37.5% had both. Median sum MFI was 6219 (range 1223–124449). Compared to recipients that did not develop anti-VA dnDSA, those who did were younger (45.7 vs 55.7 yrs), had more HLA mismatches with the vessel donor (87.5% vs 63.3% had ≥ 6/8, 6 being the median for the entire group), were more likely to have developed anti-organ dnDSA (25.0% vs 10%) and have DGF (25.0% vs 10%). Induction regimens, PRA, repeat TX, living vs deceased TX, pre-TX anti-organ and anti-VA DSA, and race and gender distributions were similar between the two groups. Cox regression modeling revealed hazard ratios of 9 (p=0.07) and 4 (p=0.18) for development of anti-VA dnDSA with higher number of HLA mismatches with vessel donor and anti-organ dnDSA respectively. One patient was diagnosed with TX renal artery stenosis 3.2 years after anti-VA dnDSA detection; no patient without anti-VA dnDSA suffered any vascular complications.
Anti-VA dnDSA developed in about one-fifth of TX recipients requiring VA. More data are required on their clinical relevance. This may be especially relevant in haploidentical TX that are routinely administered less immunosuppression.
CITATION INFORMATION: Garg N., Ellis T., Redfield R., Parajuli S., Astor B., Djamali A., Mandelbrot D. De Novo DSA against Third Party Extension Vessels Used in Kidney and Pancreas Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Garg N, Ellis T, Redfield R, Parajuli S, Astor B, Djamali A, Mandelbrot D. De Novo DSA against Third Party Extension Vessels Used in Kidney and Pancreas Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/de-novo-dsa-against-third-party-extension-vessels-used-in-kidney-and-pancreas-transplantation/. Accessed March 3, 2026.« Back to 2018 American Transplant Congress
