Critical Analysis of Valganciclovir Dosing and Renal Function on the Development of CMV Infection in Kidney Transplantation
Department of Medicine, Division of Nephrology and Hypertension, Medical University of South Carolina, Charleston, SC
Department of Pharmacy, Medical University of South Carolina, Charleston, SC
Meeting: 2013 American Transplant Congress
Abstract number: 88
Purpose: To evaluate factors that impact the development of CMV infection in renal transplant recipients.
Background: Cytomegalovirus (CMV) infection is one of the most common and important opportunistic infections following kidney transplantation. It causes significant morbidity and mortality. Previous studies report high risk CMV serostatus and increased immunosuppression as factors that increase the incidence of CMV infection.
Methods: We conducted a post-hoc analysis of a randomized controlled trial in 187 kidney transplant recipients to evaluate the factors that significantly impact the development of CMV infection.
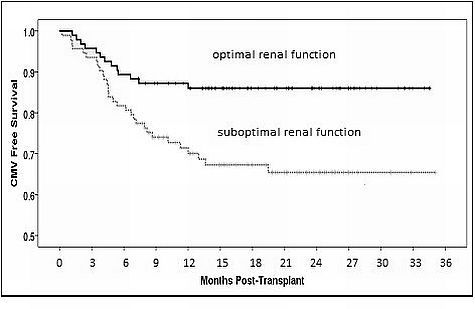
Results and Conclusion: The results demonstrate that the following variables were independent risk factors for the development of CMV infection: high risk CMV serostatus, anti-thymocyte globulin induction therapy, higher mean tacrolimus trough, creatinine clearance < 60 ml/min, and body weight >80 kg.
| Covariate | Hazard ratio | P-value |
| Wt >80 Kg | 2.1 | 0.037 |
| ATG induction | 2.1 | 0.028 |
| Tacrolimus trough | 1.4 | 0.007 |
| D+/R- CMV serostatus | 1.4 | 0.002 |
| CrCl <60 mL/min | 3.4 | 0.001 |
Based on currently recommended protocol adjusting for estimated GFR, Valganciclovir dosing was appropriate for most patients in those that did and did not develop CMV infection. These results suggest that the currently recommended dose adjustments of valganciclovir dosing based on estimated renal function may be inappropriate and under-expose patients to valganciclovir. Furthermore, estimated GFR calculated based on ideal body weight may not accurately reflect renal function, especially among overweight patients and result in under-dosing of Valganciclovir. Based on these findings, further valganciclovir pharmacokinetic analyses are warranted in overweight renal transplant recipients with moderate to severe renal dysfunction.
To cite this abstract in AMA style:
Salas MPosadas, Taber D, Chua E, Thomas B. Critical Analysis of Valganciclovir Dosing and Renal Function on the Development of CMV Infection in Kidney Transplantation [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/critical-analysis-of-valganciclovir-dosing-and-renal-function-on-the-development-of-cmv-infection-in-kidney-transplantation/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
