Creatinine Monitoring by Remote Blood Spot Testing in Pediatric Kidney Transplant Recipients
1Laboratory Medicine, Seattle Children's, Seattle
2Transplant, Seattle Children's, Seattle
3Pediatrics, University of Washington, Seattle.
Meeting: 2018 American Transplant Congress
Abstract number: B223
Keywords: Immunosuppression, Kidney transplantation, Pediatric
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Introduction: Pediatric kidney transplant patients are monitored frequently with laboratory testing to assess kidney transplant function and to optimize therapeutic drug levels. Challenges to timely collection include: rural and underserved areas, inconvenient lab hours, inconvenient timing with clinic visits, transportation costs, and elderly or busy families. Capillary Dried Blood Spots (DBS) are in use for immunosuppressive drug monitoring. In this study, we sought to establish the feasibility and accuracy of using DBS to measure creatinine in the pediatric kidney transplant population.
Methods: We collected simultaneously paired venous and finger-poke (capillary) to assess the correlation of venous plasma creatinine with capillary dried blood spot creatinine. This method uses creatinine-d3 as the calibrators, and creatinine-13C3-d3 as internal standard measured with a SCIEX QTRAP 6500. Chromatographic separation was achieved with an Acquity HSS T3 column (2.1 x 100 mm, 1.8 mcm) over 2.8 min. Limits of detection and quantitation were determined on the equivalent of 3 [micro]L dried blood spot extractions. Intra-assay and inter-assay precision at 0.25 mg/dL were 5.4% and 11.5%, respectively.
Results: A total of 48 samples via phlebotomy from 43 participants were available for analysis. We demonstrate a strong correlation between venous and capillary (DBS) creatinine values (R2 = 0.94). There is a systematic negative bias of ~30% (y = 0.71x + 0.17). This bias can be corrected by using the linear regression as a correction factor. After correction, the median bias was reduced to -0.01 mg/dL, which we determine to be clinically insignificant.
Conclusion: Measuring creatinine by remotely collected dried blood spots was shown to be clinically reliable and logistically possible in pediatric kidney transplant recipients when a correction factor was applied. Eliminating barriers to timely lab draws may improve adherence, decrease costs, and improve overall quality of life. 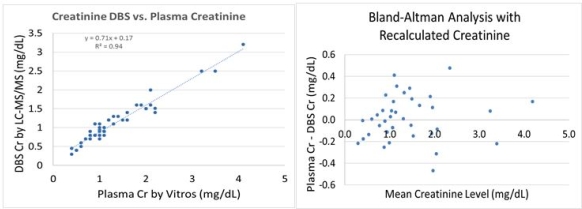
CITATION INFORMATION: Dickerson J., Jacot K., Sinkey M., Smith J. Creatinine Monitoring by Remote Blood Spot Testing in Pediatric Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Dickerson J, Jacot K, Sinkey M, Smith J. Creatinine Monitoring by Remote Blood Spot Testing in Pediatric Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/creatinine-monitoring-by-remote-blood-spot-testing-in-pediatric-kidney-transplant-recipients/. Accessed February 25, 2026.« Back to 2018 American Transplant Congress
