Correlations of In Vitro Islet Potency Tests with Clinical Islet Auto-Transplant Outcome
Surgery, University of Arizona, Tucson, AZ
Surgery, University of Minnesota, Minneapolis, MN
Schulze Diabetes Institute, Minneapolis, MN
Information Science, City of Hope, Duarte, CA
Meeting: 2013 American Transplant Congress
Abstract number: D1575
There is a critical need for real-time in vitro islet characterization assays that are predictive of clinical transplant outcomes (CTO), but none is available so far. Intraportal islet auto-transplant (IAT) in patients with chronic pancreatitis undergoing total pancreatectomy was utilized as a model for evaluating the relationship between such assays and CTO. IAT is an attractive model as it does not suffer from the presence of confounding factors, such as auto-, allo-, and xeno-immunity or immunosuppressive drug toxicity. Islet cell membrane integrity (FDA/PI), the oxygen consumption rate (OCR) normalized to DNA, islet index [number of islet equivalents (IE, sphere with a diameter of 150 micrometers)/number of islets], IE dose based on counts, and the viable IE dose (IE dose multiplied by viability as measured by OCR/DNA) were assessed in islet autograft products with purities ranging from 10% to 95% (n=35). Recipients with follow ups from 6-12 months were included in the analysis. IAT recipients with fasting blood glucose (BG) <126 mg/dL, 2-hour postprandial BG <180 mg/dL, and HbA1c ≤6.5% without administration of exogenous insulin were considered insulin independent (II). Relationships with outcomes were examined using receiver operating characteristic (ROC) analysis (Figure 1 a-e). IE and OCR dose where highly predictive of CTO. OCR dose was better than IE dose in predicting II, and helped correctly classify CTO where IE dose was high but II was not achieved or IE dose was low and II was achieved (Figure 1f). OCR/DNA, FDA/PI, or OCR/DNA/Islet index were not predictive.
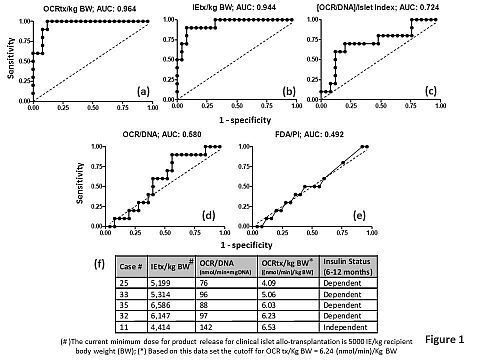
The data presented suggest that in addition to IE dose, OCR dose but not the ratio of OCR/DNA to islet index, may be useful for the prospective evaluation of the quality of islet preparations prior to clinical transplantation.
To cite this abstract in AMA style:
Papas K, Bellin M, Sutherland D, Kitzmann J, Avgoustiniatos E, Gruessner A, Mueller K, Balamurugan A, Rozak P, Loganathan G, Suszynski T, Wilhem J, Qian D, Niland J, Hering B. Correlations of In Vitro Islet Potency Tests with Clinical Islet Auto-Transplant Outcome [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/correlations-of-in-vitro-islet-potency-tests-with-clinical-islet-auto-transplant-outcome/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
