Conversion to Belatacept for Calcineurin Inhibitor Toxicities
Columbia University Medical Center, New York, NY
Meeting: 2022 American Transplant Congress
Abstract number: 416
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:40pm-4:50pm
Presentation Time: 4:40pm-4:50pm
Location: Hynes Room 302
*Purpose: Calcineurin inhibitors (CNIs) have improved renal transplant (RTx) outcomes but are associated with significant toxicities. Belatacept provides better long-term outcomes than cyclosporine, has less nephrotoxicity, but higher rejection rates. Conversion to Belatacept is one solution to patients experiencing CNI side effects. We reviewed our experience with Belatacept conversion to assess outcomes and identify predictors of response to conversion.
*Methods: We reviewed our transplant database to identify pts who were converted to Belatacept from a CNI based regimen. Donor characteristics, demographics, graft outcomes, rejections, and infectious complications were recorded.
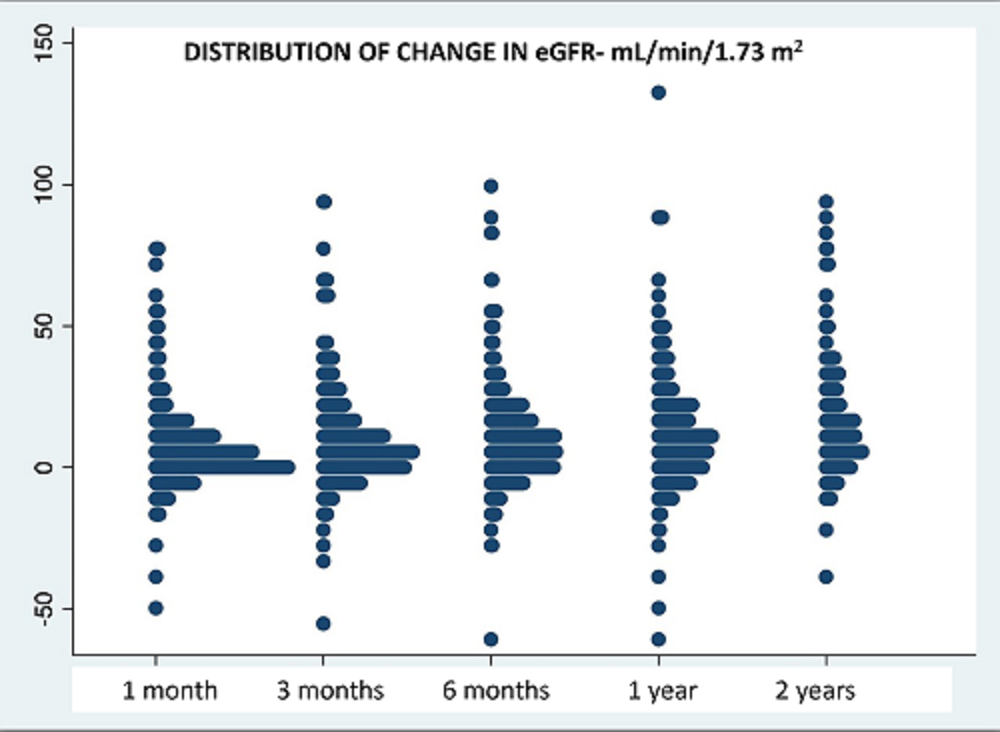
*Results: Since 2013, we have converted 380 pts to Belatacept: 287 for graft dysfunction, 25 for thrombotic microangiopathy (TMA), 59 for neurotoxicity, and 9 for other reasons. Pts were 49.6 (SD 15.2) years old, 40% female, 233 from deceased donors, and converted after 615 (SD 1064) days. At a mean follow up of 1046 (SD 793) days, eGFR improved from 33 (SD 21) cc/min to 43 (SD 17) at 3 months and to 46 (SD 23) at 2 years. Distribution of eGFR improvement at each time point is provided below (figure 1). On multivariate analysis, predictors of improvements in eGFR at 6 months included: earlier conversion, absence of vascular disease on biopsy, and lower eGFR at time of conversion. 60 pts (16%) had T cell mediated rejection (TCMR) after conversion — borderline= 25, 1A= 11, 1B=12, 2A=7, 2B=3, 3=2. 6 TCMR pts had concomitant antibody mediated rejection (AMR) and 3 pts had chronic active AMR. Graft failure occurred in 79% of rejectors versus 12% without rejection (p <0.0001). Of 272 patients without donor specific antibody (DSA) prior to conversion, 164 had repeat DSA screening, 9 (5%) of whom had de novo DSA. Urine protein:creatinine ratio was stable, 0.8 (SD 2.8) prior and 0.9 (SD 2.2) after conversion.
There were 57 episodes of CMV viremia, 35% of which broke through valganciclovir prophylaxis. Other infections were common: BKV (59), EBV (28), fungal disease (7), and adenovirus nephritis (1). 25 cancers occurred in 24 patients (9 – skin cancer, 6- lymphoma, 2- Kaposi sarcoma, 2- renal cell carcinoma, 1 each: acute leukemia, breast, colon, lung, pancreas, rectal). In total, there were 36 deaths and 46 graft failures.
*Conclusions: Conversion to belatacept improved renal function in many patients with rejection rates similar to what has been previously described.* Close attention needs to be paid towards appropriate anti-viral prophylaxis and prompt detection of rejection to avoid adverse outcomes. *Grinyo J. et al. Am J Kidney Dis 2017, 69(5):587-594
To cite this abstract in AMA style:
Patel SS, Jadav P, Sekulic M, Kudose S, Batal I, Mohan S, Crew R. Conversion to Belatacept for Calcineurin Inhibitor Toxicities [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-to-belatacept-for-calcineurin-inhibitor-toxicities/. Accessed February 22, 2026.« Back to 2022 American Transplant Congress