Conversion to Belatacept as Rescue Therapy in Kidney Transplant Recipients with Severe Graft Dysfunction.
1Clinic for General, Abdominal and Transplant Surgery, Hannover Medical School, Hannover, Germany
2Clinic for Nephrology, Hannover Medical School, Hannover, Germany.
Meeting: 2016 American Transplant Congress
Abstract number: D142
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Belatacept is a fusion protein that prevents T cell co-stimulation via the CD28 receptor. If used as first-line therapy, immunosuppression with Belatacept is associated with improved renal function compared to CNI-based immunosuppressive regimens.
Here we present our experience using Belatacept as rescue therapy in kidney transplant patients with severely impaired graft function. We selected patients with deterioration in function (GFR < 30 ml/min), with no sign of rejection but severe chronic histologic damage (IF/TA +/- chronic vascular changes) in the biopsy who received CNI-based immunosuppression or who were unable to continue CNI or mTOR based immunosuppression due to severe side effects. We hypothesized that the discontinuation of CNI or mTOR and switch to Belatacept would improve and stabilize graft function even at this advanced stage of allograft damage.
Sixteen (mean age 50 ± 20 years) patients were switched from CNIs or mTORs to belatacept at a mean of 63 ± 87 months post transplantation. Renal function improved from a mean estimated glomerular filtration rate (eGFR) of 20.8 ± 9.4 mL/min/1.73 m2 prior to the switch to a eGFR of 23.9 ± 12.7 mL/min/1.73 m2 3 months after the conversion (p = 0.04). 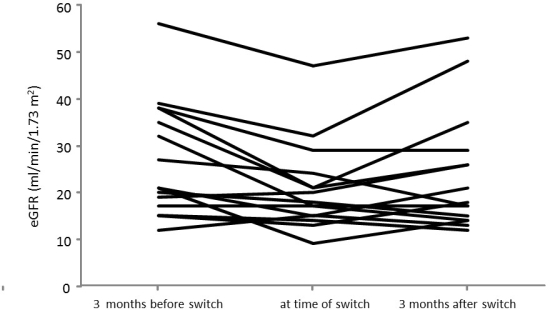 In 5/16 patients we could not achieve improvement of allograft function. Most patients tolerated the conversion well; only 2 patients developed clinically relevant side effects: one patient developed a pneumocystis pneumonia; in one patient belatacept had to be discontinued due to severe leucopenia. One patient developed an acute cellular rejection shortly after the conversion.
In 5/16 patients we could not achieve improvement of allograft function. Most patients tolerated the conversion well; only 2 patients developed clinically relevant side effects: one patient developed a pneumocystis pneumonia; in one patient belatacept had to be discontinued due to severe leucopenia. One patient developed an acute cellular rejection shortly after the conversion.
Our data shows that belatacept can be used as a rescue therapy in patients with severely impaired graft function and chronic histologic damage. However further studies are necessary to establish the long term effect and define the indication for a conversion to a belatacept based immunosuppresive therapy.
CITATION INFORMATION: Oldhafer F, Hiss M, Schwarz A, Haller H, Einecke G. Conversion to Belatacept as Rescue Therapy in Kidney Transplant Recipients with Severe Graft Dysfunction. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Oldhafer F, Hiss M, Schwarz A, Haller H, Einecke G. Conversion to Belatacept as Rescue Therapy in Kidney Transplant Recipients with Severe Graft Dysfunction. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-to-belatacept-as-rescue-therapy-in-kidney-transplant-recipients-with-severe-graft-dysfunction/. Accessed March 2, 2026.« Back to 2016 American Transplant Congress
