Conversion to a Belatacept Based Immunosuppression Regimen in Renal Transplant Recipients
Northwestern Memorial Hospital, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: 116
Keywords: Antibodies, Immunosuppression, Kidney
Session Information
Session Name: Novel Immunosuppression: Belatacept
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:27pm-4:39pm
Presentation Time: 4:27pm-4:39pm
Location: Virtual
*Purpose: Belatacept, a co-stimulation blocker, is an alternative anti-rejection agent that may avoid nephrotoxic or intolerable adverse effects of calcineurin inhibitor (CNI) or mammalian target of rapamycin inhibitor (mTORi) based regimens and has shown to preserve long-term renal function. Recent data has demonstrated improvement or stabilization of pre-existing donor specific antibodies (DSA) in belatacept treated patients. This study evaluates the impact of conversion to belatacept on renal function and DSA.
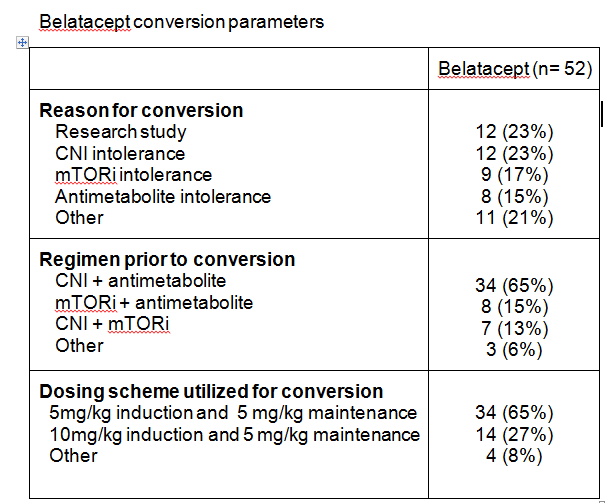
*Methods: This is a single center, retrospective review of adult renal transplant recipients converted to belatacept. Patients were excluded if they received a multi-organ transplant or did not reach maintenance dosing for belatacept. Trends in DSA and eGFR at 12 months after conversion were assessed.
*Results: Fifty-two patients were analyzed. Median time to belatacept conversion was 427 days post-transplant (IQR 98-525). Serum creatinine changed from a mean 2.1±1.5mg/dL at time of conversion to 1.6±0.6mg/dL at 12 months (P=0.0675). Mean eGFR at time of conversion was 44.1±23.2ml/min and changed to 51.1±21.7ml/min at month 12 post-conversion (P=0.1422). Rejection occurred in 17 patients (32.7%) post conversion. Sixteen experienced borderline rejection; 4 of which were treated with steroids. One patient experienced Banff 1A rejection and was treated with steroids. No patients developed PTLD within one year of conversion. Four patients had detectable DSA prior to transplant with 6 additional patients developing de novo DSA post-transplant but prior to belatacept conversion. Nine of 10 patients with DSA prior to belatacept conversion experienced either decreased DSA titers or DSA stabilization after initiating belatacept. No patients developed de novo DSA post-belatacept conversion.
*Conclusions: Conversion to belatacept was associated with a similar eGFR at 12 months in kidney transplant patients who had previously been on CNI or mTORi based immunosuppression. Following belatacept conversion, patients with detectable DSA were found to either have decreased or stabilization of DSA titers. Conversion to belatacept may be a desirable option for patients who have intolerance to CNI or mTORi based regimens. Analysis in a larger number of patients should be evaluated.
To cite this abstract in AMA style:
Paplaczyk K, Cunningham K, D'Agostino C, Pinelli D, Schulte J, Shetty A. Conversion to a Belatacept Based Immunosuppression Regimen in Renal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-to-a-belatacept-based-immunosuppression-regimen-in-renal-transplant-recipients/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress